A Potential Role for a Genetic Variation of AKAP5 in Human Aggression and Anger Control
- PMID: 22232585
- PMCID: PMC3247758
- DOI: 10.3389/fnhum.2011.00175
A Potential Role for a Genetic Variation of AKAP5 in Human Aggression and Anger Control
Abstract
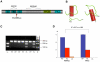
The A-kinase-anchoring protein 5 (AKAP5), a post-synaptic multi-adaptor molecule that binds G-protein-coupled receptors and intracellular signaling molecules has been implicated in emotional processing in rodents, but its role in human emotion and behavior is up to now still not quite clear. Here, we report an association of individual differences in aggressive behavior and anger expression with a functional genetic polymorphism (Pro100Leu) in the human AKAP5 gene. Among a cohort of 527 young, healthy individuals, carriers of the less common Leu allele (15.6% allele frequency) scored significantly lower in the physical aggression domain of the Buss and Perry Aggression Questionnaire and higher in the anger control dimension of the state-trait anger expression inventory. In a functional magnetic resonance imaging experiment we could further demonstrate that AKAP5 Pro100Leu modulates the interaction of negative emotional processing and executive functions. In order to investigate implicit processes of anger control, we used the well-known flanker task to evoke processes of action monitoring and error processing and added task-irrelevant neutral or angry faces in the background of the flanker stimuli. In line with our predictions, Leu carriers showed increased activation of the anterior cingulate cortex (ACC) during emotional interference, which in turn predicted shorter reaction times and might be related to stronger control of emotional interference. Conversely, Pro homozygotes exhibited increased orbitofrontal cortex (OFC) activation during emotional interference, with no behavioral advantage. Immunohistochemistry revealed AKAP5 expression in post mortem human ACC and OFC. Our results suggest that AKAP5 Pro100Leu contributes to individual differences in human aggression and anger control. Further research is warranted to explore the detailed role of AKAP5 and its gene product in human emotion processing.
Keywords: AKAP5; aggression; anger; fMRI; genetic.
Figures



Similar articles
-
Effects of AKAP5 Pro100Leu genotype on working memory for emotional stimuli.PLoS One. 2013;8(1):e55613. doi: 10.1371/journal.pone.0055613. Epub 2013 Jan 29. PLoS One. 2013. PMID: 23383244 Free PMC article.
-
Amygdala and orbitofrontal reactivity to social threat in individuals with impulsive aggression.Biol Psychiatry. 2007 Jul 15;62(2):168-78. doi: 10.1016/j.biopsych.2006.08.024. Epub 2007 Jan 8. Biol Psychiatry. 2007. PMID: 17210136
-
Whole-brain functional connectivity during script-driven aggression in borderline personality disorder.Prog Neuropsychopharmacol Biol Psychiatry. 2019 Jul 13;93:46-54. doi: 10.1016/j.pnpbp.2019.03.004. Epub 2019 Mar 16. Prog Neuropsychopharmacol Biol Psychiatry. 2019. PMID: 30885789
-
Neural functional correlates of the impact of socio-emotional stimuli on performances on a flanker task in children aged 9-11 years.Neuropsychologia. 2020 Aug;145:106747. doi: 10.1016/j.neuropsychologia.2018.04.004. Epub 2018 Apr 5. Neuropsychologia. 2020. PMID: 29627273
-
Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies.J Psychiatry Neurosci. 2009 Nov;34(6):418-32. J Psychiatry Neurosci. 2009. PMID: 19949718 Free PMC article. Review.
Cited by
-
Motivational salience and genetic variability of dopamine D2 receptor expression interact in the modulation of interference processing.Front Hum Neurosci. 2013 Jun 5;7:250. doi: 10.3389/fnhum.2013.00250. eCollection 2013. Front Hum Neurosci. 2013. PMID: 23760450 Free PMC article.
-
Polymorphisms/Mutations in A-Kinase Anchoring Proteins (AKAPs): Role in the Cardiovascular System.J Cardiovasc Dev Dis. 2018 Jan 25;5(1):7. doi: 10.3390/jcdd5010007. J Cardiovasc Dev Dis. 2018. PMID: 29370121 Free PMC article. Review.
-
Identification of the common neurobiological process disturbed in genetic and non-genetic models for autism spectrum disorders.Cell Mol Life Sci. 2022 Nov 13;79(12):589. doi: 10.1007/s00018-022-04617-3. Cell Mol Life Sci. 2022. PMID: 36371739 Free PMC article.
-
Genetic variation of the RASGRF1 regulatory region affects human hippocampus-dependent memory.Front Hum Neurosci. 2014 Apr 29;8:260. doi: 10.3389/fnhum.2014.00260. eCollection 2014. Front Hum Neurosci. 2014. PMID: 24808846 Free PMC article.
-
Effects of Repetitive Mild Traumatic Brain Injury on Corticotropin-Releasing Factor Modulation of Lateral Habenula Excitability and Motivated Behavior.bioRxiv [Preprint]. 2024 May 14:2024.04.16.589760. doi: 10.1101/2024.04.16.589760. bioRxiv. 2024. Update in: J Neurotrauma. 2025 May;42(9-10):832-850. doi: 10.1089/neu.2024.0184. PMID: 38798343 Free PMC article. Updated. Preprint.
References
-
- Bertolino A., Rubino V., Sambataro F., Blasi G., Latorre V., Fazio L., Caforio G., Petruzzella V., Kolachana B., Hariri A., Meyer-Lindenberg A., Nardini M., Weinberger D. R., Scarabino T. (2006). Prefrontal-hippocampal coupling during memory processing is modulated by COMT val158met genotype. Biol. Psychiatry 60, 1250–125810.1016/j.biopsych.2006.03.078 - DOI - PubMed
-
- Blasi G., Mattay V. S., Bertolino A., Elvevag B., Callicott J. H., Das S., Kolachana B. S., Egan M. F., Goldberg T. E., Weinberger D. R. (2005). Effect of catechol-O-methyltransferase val158met genotype on attentional control. J. Neurosci. 25, 5038–504510.1523/JNEUROSCI.0476-05.2005 - DOI - PMC - PubMed

