High-resolution fiber tract reconstruction in the human brain by means of three-dimensional polarized light imaging
- PMID: 22232597
- PMCID: PMC3248698
- DOI: 10.3389/fninf.2011.00034
High-resolution fiber tract reconstruction in the human brain by means of three-dimensional polarized light imaging
Abstract
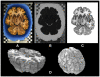
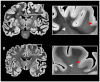
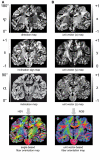
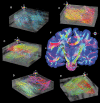
Functional interactions between different brain regions require connecting fiber tracts, the structural basis of the human connectome. To assemble a comprehensive structural understanding of neural network elements from the microscopic to the macroscopic dimensions, a multimodal and multiscale approach has to be envisaged. However, the integration of results from complementary neuroimaging techniques poses a particular challenge. In this paper, we describe a steadily evolving neuroimaging technique referred to as three-dimensional polarized light imaging (3D-PLI). It is based on the birefringence of the myelin sheaths surrounding axons, and enables the high-resolution analysis of myelinated axons constituting the fiber tracts. 3D-PLI provides the mapping of spatial fiber architecture in the postmortem human brain at a sub-millimeter resolution, i.e., at the mesoscale. The fundamental data structure gained by 3D-PLI is a comprehensive 3D vector field description of fibers and fiber tract orientations - the basis for subsequent tractography. To demonstrate how 3D-PLI can contribute to unravel and assemble the human connectome, a multiscale approach with the same technology was pursued. Two complementary state-of-the-art polarimeters providing different sampling grids (pixel sizes of 100 and 1.6 μm) were used. To exemplarily highlight the potential of this approach, fiber orientation maps and 3D fiber models were reconstructed in selected regions of the brain (e.g., Corpus callosum, Internal capsule, Pons). The results demonstrate that 3D-PLI is an ideal tool to serve as an interface between the microscopic and macroscopic levels of organization of the human connectome.
Keywords: PLI; U-fiber; connectome; human brain; method; polarized light imaging; systems biology; white matter.
Figures




References
-
- Amunts K., Zilles K. (2006). “Atlases of the human brain: tools for functional neuroimaging,” in Neuroanatomical Tract Tracing 3: Molecules, Neurons, and Systems, eds Zaborsky L., Wouterlood F. G., Lanciego J. L. (New York: Springer; ), 566–603
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

