Translational inhibition by deadenylation-independent mechanisms is central to microRNA-mediated silencing in zebrafish
- PMID: 22232654
- PMCID: PMC3268308
- DOI: 10.1073/pnas.1113350109
Translational inhibition by deadenylation-independent mechanisms is central to microRNA-mediated silencing in zebrafish
Abstract
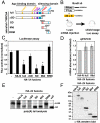
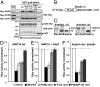
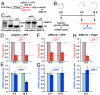
MicroRNA (miRNA) is a class of small noncoding RNA approximately 22 nt in length. Animal miRNA silences complementary mRNAs via translational inhibition, deadenylation, and mRNA degradation. However, the underlying molecular mechanisms remain unclear. A key question is whether these three outputs are independently induced by miRNA through distinct mechanisms or sequentially induced within a single molecular pathway. Here, we successfully dissected these intricate outputs of miRNA-mediated repression using zebrafish embryos as a model system. Our results indicate that translational inhibition and deadenylation are independent outputs mediated by distinct domains of TNRC6A, which is an effector protein in the miRNA pathway. Translational inhibition by TNRC6A is divided into two mechanisms: PAM2 motif-mediated interference of poly(A)-binding protein (PABP), and inhibition of 5' cap- and poly(A) tail-independent step(s) by a previously undescribed P-GL motif. Consistent with these observations, we show that, in zebrafish embryos, miRNA inhibits translation of the target mRNA in a deadenylation- and PABP-independent manner at early time points. These results indicate that miRNA exerts multiple posttranscriptional outputs via physically and functionally independent mechanisms and that direct translational inhibition is central to miRNA-mediated repression.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Bushati N, Cohen SM. microRNA functions. Annu Rev Cell Dev Biol. 2007;23:175–205. - PubMed
-
- Fabian MR, Sonenberg N, Filipowicz W. Regulation of mRNA translation and stability by microRNAs. Annu Rev Biochem. 2010;79:351–379. - PubMed
-
- Lim LP, et al. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature. 2005;433:769–773. - PubMed
-
- Giraldez AJ, et al. Zebrafish MiR-430 promotes deadenylation and clearance of maternal mRNAs. Science. 2006;312:75–79. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

