Brain structure in healthy adults is related to serum transferrin and the H63D polymorphism in the HFE gene
- PMID: 22232660
- PMCID: PMC3325658
- DOI: 10.1073/pnas.1105543109
Brain structure in healthy adults is related to serum transferrin and the H63D polymorphism in the HFE gene
Abstract
Control of iron homeostasis is essential for healthy central nervous system function: iron deficiency is associated with cognitive impairment, yet iron overload is thought to promote neurodegenerative diseases. Specific genetic markers have been previously identified that influence levels of transferrin, the protein that transports iron throughout the body, in the blood and brain. Here, we discovered that transferrin levels are related to detectable differences in the macro- and microstructure of the living brain. We collected brain MRI scans from 615 healthy young adult twins and siblings, of whom 574 were also scanned with diffusion tensor imaging at 4 Tesla. Fiber integrity was assessed by using the diffusion tensor imaging-based measure of fractional anisotropy. In bivariate genetic models based on monozygotic and dizygotic twins, we discovered that partially overlapping additive genetic factors influenced transferrin levels and brain microstructure. We also examined common variants in genes associated with transferrin levels, TF and HFE, and found that a commonly carried polymorphism (H63D at rs1799945) in the hemochromatotic HFE gene was associated with white matter fiber integrity. This gene has a well documented association with iron overload. Our statistical maps reveal previously unknown influences of the same gene on brain microstructure and transferrin levels. This discovery may shed light on the neural mechanisms by which iron affects cognition, neurodevelopment, and neurodegeneration.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
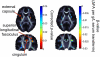



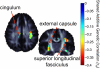




References
-
- CDC From the Centers for Disease Control and Prevention. Iron deficiency—United States, 1999-2000. JAMA. 2002;288:2114–2116. - PubMed
-
- Halterman JS, Kaczorowski JM, Aligne CA, Auinger P, Szilagyi PG. Iron deficiency and cognitive achievement among school-aged children and adolescents in the United States. Pediatrics. 2001;107:1381–1386. - PubMed
-
- Nelson C, Erikson K, Piñero DJ, Beard JL. In vivo dopamine metabolism is altered in iron-deficient anemic rats. J Nutr. 1997;127:2282–2288. - PubMed
-
- Bartzokis G, et al. In vivo evaluation of brain iron in Alzheimer's disease and normal subjects using MRI. Biol Psychiatry. 1994;35:480–487. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

