Hypoxic regulation of the cerebral microcirculation is mediated by a carbon monoxide-sensitive hydrogen sulfide pathway
- PMID: 22232681
- PMCID: PMC3268316
- DOI: 10.1073/pnas.1119658109
Hypoxic regulation of the cerebral microcirculation is mediated by a carbon monoxide-sensitive hydrogen sulfide pathway
Abstract
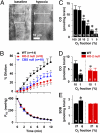
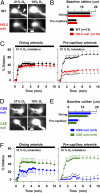
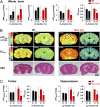
Enhancement of cerebral blood flow by hypoxia is critical for brain function, but signaling systems underlying its regulation have been unclear. We report a pathway mediating hypoxia-induced cerebral vasodilation in studies monitoring vascular disposition in cerebellar slices and in intact mouse brains using two-photon intravital laser scanning microscopy. In this cascade, hypoxia elicits cerebral vasodilation via the coordinate actions of H(2)S formed by cystathionine β-synthase (CBS) and CO generated by heme oxygenase (HO)-2. Hypoxia diminishes CO generation by HO-2, an oxygen sensor. The constitutive CO physiologically inhibits CBS, and hypoxia leads to increased levels of H(2)S that mediate the vasodilation of precapillary arterioles. Mice with targeted deletion of HO-2 or CBS display impaired vascular responses to hypoxia. Thus, in intact adult brain cerebral cortex of HO-2-null mice, imaging mass spectrometry reveals an impaired ability to maintain ATP levels on hypoxia.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Iadecola C, Nedergaard M. Glial regulation of the cerebral microvasculature. Nat Neurosci. 2007;10:1369–1376. - PubMed
-
- Ances BM. Coupling of changes in cerebral blood flow with neural activity: What must initially dip must come back up. J Cereb Blood Flow Metab. 2004;24:1–6. - PubMed
-
- Hoffmeyer HW, Enager P, Thomsen KJ, Lauritzen MJ. Nonlinear neurovascular coupling in rat sensory cortex by activation of transcallosal fibers. J Cereb Blood Flow Metab. 2007;27:575–587. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

