How Staphylococcus aureus biofilms develop their characteristic structure
- PMID: 22232686
- PMCID: PMC3268330
- DOI: 10.1073/pnas.1115006109
How Staphylococcus aureus biofilms develop their characteristic structure
Abstract
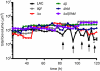
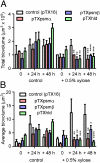
Biofilms cause significant problems in the environment and during the treatment of infections. However, the molecular mechanisms underlying biofilm formation are poorly understood. There is a particular lack of knowledge about biofilm maturation processes, such as biofilm structuring and detachment, which are deemed crucial for the maintenance of biofilm viability and the dissemination of cells from a biofilm. Here, we identify the phenol-soluble modulin (PSM) surfactant peptides as key biofilm structuring factors in the premier biofilm-forming pathogen Staphylococcus aureus. We provide evidence that all known PSM classes participate in structuring and detachment processes. Specifically, absence of PSMs in isogenic S. aureus psm deletion mutants led to strongly impaired formation of biofilm channels, abolishment of the characteristic waves of biofilm detachment and regrowth, and loss of control of biofilm expansion. In contrast, induced expression of psm loci in preformed biofilms promoted those processes. Furthermore, PSMs facilitated dissemination from an infected catheter in a mouse model of biofilm-associated infection. Moreover, formation of the biofilm structure was linked to strongly variable, quorum sensing-controlled PSM expression in biofilm microenvironments, whereas overall PSM production remained constant to ascertain biofilm homeostasis. Our study describes a mechanism of biofilm structuring in molecular detail, and the general principle (i.e., quorum-sensing controlled expression of surfactants) seems to be conserved in several bacteria, despite the divergence of the respective biofilm-structuring surfactants. These findings provide a deeper understanding of biofilm development processes, which represents an important basis for strategies to interfere with biofilm formation in the environment and human disease.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



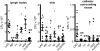
Comment in
-
Biofilms: Biofilms take shape.Nat Rev Microbiol. 2012 Feb 13;10(3):162. doi: 10.1038/nrmicro2756. Nat Rev Microbiol. 2012. PMID: 22330879 No abstract available.
References
-
- Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: A common cause of persistent infections. Science. 1999;284:1318–1322. - PubMed
-
- Vuong C, Saenz HL, Götz F, Otto M. Impact of the agr quorum-sensing system on adherence to polystyrene in Staphylococcus aureus. J Infect Dis. 2000;182:1688–1693. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

