The septin cytoskeleton facilitates membrane retraction during motility and blebbing
- PMID: 22232702
- PMCID: PMC3255977
- DOI: 10.1083/jcb.201105127
The septin cytoskeleton facilitates membrane retraction during motility and blebbing
Abstract
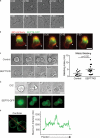
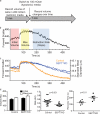
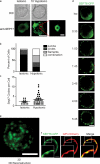
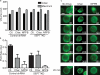
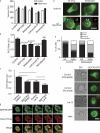
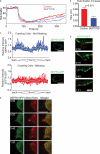
Increasing evidence supports a critical role for the septin cytoskeleton at the plasma membrane during physiological processes including motility, formation of dendritic spines or cilia, and phagocytosis. We sought to determine how septins regulate the plasma membrane, focusing on this cytoskeletal element's role during effective amoeboid motility. Surprisingly, septins play a reactive rather than proactive role, as demonstrated during the response to increasing hydrostatic pressure and subsequent regulatory volume decrease. In these settings, septins were required for rapid cortical contraction, and SEPT6-GFP was recruited into filaments and circular patches during global cortical contraction and also specifically during actin filament depletion. Recruitment of septins was also evident during excessive blebbing initiated by blocking membrane trafficking with a dynamin inhibitor, providing further evidence that septins are recruited to facilitate retraction of membranes during dynamic shape change. This function of septins in assembling on an unstable cortex and retracting aberrantly protruding membranes explains the excessive blebbing and protrusion observed in septin-deficient T cells.
Figures
References
-
- Carton I., Hermans D., Eggermont J. 2003. Hypotonicity induces membrane protrusions and actin remodeling via activation of small GTPases Rac and Cdc42 in Rat-1 fibroblasts. Am. J. Physiol. Cell Physiol. 285:C935–C944 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

