A novel function for Cyclin A2: control of cell invasion via RhoA signaling
- PMID: 22232705
- PMCID: PMC3255987
- DOI: 10.1083/jcb.201102085
A novel function for Cyclin A2: control of cell invasion via RhoA signaling
Abstract
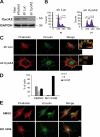
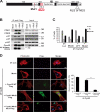
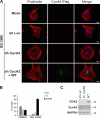
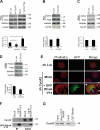
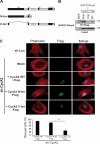
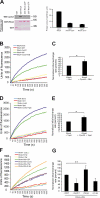
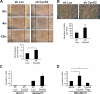
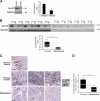
Cyclin A2 plays a key role in cell cycle regulation. It is essential in embryonic cells and in the hematopoietic lineage yet dispensable in fibroblasts. In this paper, we demonstrate that Cyclin A2-depleted cells display a cortical distribution of actin filaments and increased migration. These defects are rescued by restoration of wild-type Cyclin A2, which directly interacts with RhoA, or by a Cyclin A2 mutant unable to associate with Cdk. In vitro, Cyclin A2 potentiates the exchange activity of a RhoA-specific guanine nucleotide exchange factor. Consistent with this, Cyclin A2 depletion enhances migration of fibroblasts and invasiveness of transformed cells via down-regulation of RhoA activity. Moreover, Cyclin A2 expression is lower in metastases relative to primary colon adenocarcinoma in matched human tumors. All together, these data show that Cyclin A2 negatively controls cell motility by promoting RhoA activation, thus demonstrating a novel Cyclin A2 function in cytoskeletal rearrangements and cell migration.
Figures
Comment in
-
To be or not to be a proliferation marker?Oncogene. 2014 Feb 20;33(8):954-5. doi: 10.1038/onc.2013.19. Epub 2013 Feb 11. Oncogene. 2014. PMID: 23396366
References
-
- Aaltomaa S., Lipponen P., Ala-Opas M., Eskelinen M., Syrjänen K., Kosma V.M. 1999. Expression of cyclins A and D and p21(waf1/cip1) proteins in renal cell cancer and their relation to clinicopathological variables and patient survival. Br. J. Cancer. 80:2001–2007 10.1038/sj.bjc.6690634 - DOI - PMC - PubMed
-
- Bergmann-Leitner E.S., Abrams S.I. 2000. Differential role of Fas/Fas ligand interactions in cytolysis of primary and metastatic colon carcinoma cell lines by human antigen-specific CD8+ CTL. J. Immunol. 164:4941–4954 - PubMed
-
- Bondi J., Husdal A., Bukholm G., Nesland J.M., Bakka A., Bukholm I.R. 2005. Expression and gene amplification of primary (A, B1, D1, D3, and E) and secondary (C and H) cyclins in colon adenocarcinomas and correlation with patient outcome. J. Clin. Pathol. 58:509–514 10.1136/jcp.2004.020347 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

