The Effect of Doxycycline on PMA-Induced MUC5B Expression via MMP-9 and p38 in NCI-H292 Cells
- PMID: 22232712
- PMCID: PMC3250581
- DOI: 10.3342/ceo.2011.4.4.177
The Effect of Doxycycline on PMA-Induced MUC5B Expression via MMP-9 and p38 in NCI-H292 Cells
Abstract
Objectives: Doxycycline is commonly used in medicine for its bacteriostatic antimicrobial properties. Recent studies have reported that doxycycline also has anti-inflammatory effects. Matrix metalloproteinase (MMP)-9 has been found to be involved in the physiological and pathological process of inflammatory airway disease. Phorbol 12-myristate 13-acetate (PMA), a protein kinase C activator, is known to stimulate the expression of MMP and mucin genes in the airway and intestinal epithelial cells. Therefore, the effects and signal pathways of doxycycline on PMA-induced MUC5B expression dependent MMP-9 in human airway epithelial cells were investigated.
Methods: In human NCI-H292 airway epithelial cells, MUC5B and MMP-9 mRNA expression, MUC5B protein expression, and MMP-9 protein activity after the treatment with PMA, MMP-9 or doxycycline were determined by reverse transcriptase-polymerase chain reaction, enzyme immunoassay, gelatin zymography, and Western blot analysis.
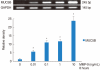
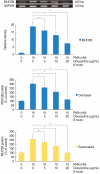
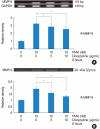
Results: PMA increased MMP-9 and MUC5B expression. MMP-9 increased MUC5B expression. Doxycycline inhibited PMA-induced MUC5B expression, and PMA-induced MMP-9 mRNA expression and protein activity. Doxycycline inhibited phosphorylation of p38 induced by PMA and MMP-9.
Conclusion: The results of this study suggest that doxycycline inhibited PMA-induced MUC5B mRNA expression and protein production through the MMP-9 and p38 pathways in human NCI-H292 airway epithelial cells.
Keywords: Doxycycline; Epithelial cells; Inflammation; MUC5B; Matrix metalloproteinase-9; Mucins; NCI-H292 cell; Phorbol myristate acetate; p38.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures

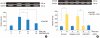

Similar articles
-
Effect of Epigallocatechin-3-Gallate on PMA-Induced MUC5B Expression in Human Airway Epithelial Cells.Clin Exp Otorhinolaryngol. 2013 Dec;6(4):237-42. doi: 10.3342/ceo.2013.6.4.237. Epub 2013 Nov 29. Clin Exp Otorhinolaryngol. 2013. PMID: 24353864 Free PMC article.
-
Phorbol 12-Myristate 13-Acetate Induces MUC16 Expression via PKCδ and p38 in Human Airway Epithelial Cells.Clin Exp Otorhinolaryngol. 2012 Sep;5(3):161-9. doi: 10.3342/ceo.2012.5.3.161. Epub 2012 Aug 27. Clin Exp Otorhinolaryngol. 2012. PMID: 22977714 Free PMC article.
-
AMPK induces MUC5B expression via p38 MAPK in NCI-H292 airway epithelial cells.Biochem Biophys Res Commun. 2011 Jun 17;409(4):669-74. doi: 10.1016/j.bbrc.2011.05.062. Epub 2011 May 17. Biochem Biophys Res Commun. 2011. PMID: 21619869
-
Staphylococcus enterotoxin A induces MUC5B expression via Toll-like receptor 2, extracellular signal-regulated kinase 1/2, and p38 mitogen-activated protein kinase in human airway epithelial cells.Am J Rhinol Allergy. 2014 Jan-Feb;28(1):e25-30. doi: 10.2500/ajra.2014.28.3971. Am J Rhinol Allergy. 2014. PMID: 24717875
Cited by
-
Enriched Astaxanthin Extract from Haematococcus pluvialis Augments Growth Factor Secretions to Increase Cell Proliferation and Induces MMP1 Degradation to Enhance Collagen Production in Human Dermal Fibroblasts.Int J Mol Sci. 2016 Jun 16;17(6):955. doi: 10.3390/ijms17060955. Int J Mol Sci. 2016. PMID: 27322248 Free PMC article.
-
Effect of Epigallocatechin-3-Gallate on PMA-Induced MUC5B Expression in Human Airway Epithelial Cells.Clin Exp Otorhinolaryngol. 2013 Dec;6(4):237-42. doi: 10.3342/ceo.2013.6.4.237. Epub 2013 Nov 29. Clin Exp Otorhinolaryngol. 2013. PMID: 24353864 Free PMC article.
References
-
- Ali MS, Pearson JP. Upper airway mucin gene expression: a review. Laryngoscope. 2007 May;117(5):932–938. - PubMed
-
- Caramori G, Casolari P, Di Gregorio C, Saetta M, Baraldo S, Boschetto P, et al. MUC5AC expression is increased in bronchial submucosal glands of stable COPD patients. Histopathology. 2009 Sep;55(3):321–331. - PubMed
-
- Turner J, Jones CE. Regulation of mucin expression in respiratory diseases. Biochem Soc Trans. 2009 Aug;37(Pt 4):877–881. - PubMed
-
- Ohbayashi H, Shimokata K. Matrix metalloproteinase-9 and airway remodeling in asthma. Curr Drug Targets Inflamm Allergy. 2005 Apr;4(2):177–181. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

