A selective and potent CXCR3 antagonist SCH 546738 attenuates the development of autoimmune diseases and delays graft rejection
- PMID: 22233170
- PMCID: PMC3298469
- DOI: 10.1186/1471-2172-13-2
A selective and potent CXCR3 antagonist SCH 546738 attenuates the development of autoimmune diseases and delays graft rejection
Abstract
Background: The CXCR3 receptor and its three interferon-inducible ligands (CXCL9, CXCL10 and CXCL11) have been implicated as playing a central role in directing a Th1 inflammatory response. Recent studies strongly support that the CXCR3 receptor is a very attractive therapeutic target for treating autoimmune diseases, such as rheumatoid arthritis, multiple sclerosis and psoriasis, and to prevent transplant rejection. We describe here the in vitro and in vivo pharmacological characterizations of a novel and potent small molecule CXCR3 antagonist, SCH 546738.
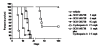
Results: In this study, we evaluated in vitro pharmacological properties of SCH 546738 by radioligand receptor binding and human activated T cell chemotaxis assays. In vivo efficacy of SCH 546738 was determined by mouse collagen-induced arthritis, rat and mouse experimental autoimmune encephalomyelitis, and rat cardiac transplantation models. We show that SCH 546738 binds to human CXCR3 with a high affinity of 0.4 nM. In addition, SCH 546738 displaces radiolabeled CXCL10 and CXCL11 from human CXCR3 with IC50 ranging from 0.8 to 2.2 nM in a non-competitive manner. SCH 546738 potently and specifically inhibits CXCR3-mediated chemotaxis in human activated T cells with IC90 about 10 nM. SCH 546738 attenuates the disease development in mouse collagen-induced arthritis model. SCH 546738 also significantly reduces disease severity in rat and mouse experimental autoimmune encephalomyelitis models. Furthermore, SCH 546738 alone achieves dose-dependent prolongation of rat cardiac allograft survival. Most significantly, SCH 546738 in combination with CsA supports permanent engraftment.
Conclusions: SCH 546738 is a novel, potent and non-competitive small molecule CXCR3 antagonist. It is efficacious in multiple preclinical disease models. These results demonstrate that therapy with CXCR3 antagonists may serve as a new strategy for treatment of autoimmune diseases, including rheumatoid arthritis and multiple sclerosis, and to prevent transplant rejection.
Figures
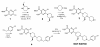






Similar articles
-
Antagonism of the chemokine receptors CXCR3 and CXCR4 reduces the pathology of experimental autoimmune encephalomyelitis.Brain Pathol. 2008 Oct;18(4):504-16. doi: 10.1111/j.1750-3639.2008.00154.x. Epub 2008 Apr 11. Brain Pathol. 2008. PMID: 18422759 Free PMC article.
-
Prolongation of cardiac and islet allograft survival by a blocking hamster anti-mouse CXCR3 monoclonal antibody.Transplantation. 2008 Jul 15;86(1):137-47. doi: 10.1097/TP.0b013e31817b8e4b. Transplantation. 2008. PMID: 18622291 Free PMC article.
-
Cross reactivity of three T cell attracting murine chemokines stimulating the CXC chemokine receptor CXCR3 and their induction in cultured cells and during allograft rejection.Eur J Immunol. 2001 Aug;31(8):2521-7. doi: 10.1002/1521-4141(200108)31:8<2521::aid-immu2521>3.0.co;2-q. Eur J Immunol. 2001. PMID: 11500837
-
Chemokine receptor CXCR3: an unexpected enigma.Curr Top Dev Biol. 2005;68:149-81. doi: 10.1016/S0070-2153(05)68006-4. Curr Top Dev Biol. 2005. PMID: 16124999 Review.
-
Chemokine (C-X-C motif) ligand (CXCL)10 in autoimmune diseases.Autoimmun Rev. 2014 Mar;13(3):272-80. doi: 10.1016/j.autrev.2013.10.010. Epub 2013 Nov 2. Autoimmun Rev. 2014. PMID: 24189283 Review.
Cited by
-
Establishing Classification Tree Models in Rheumatoid Arthritis Using Combination of Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry and Magnetic Beads.Front Med (Lausanne). 2021 Feb 24;8:609773. doi: 10.3389/fmed.2021.609773. eCollection 2021. Front Med (Lausanne). 2021. PMID: 33718399 Free PMC article.
-
Expression of CXCR3 and its ligands CXCL9, -10 and -11 in paediatric opsoclonus-myoclonus syndrome.Clin Exp Immunol. 2013 Jun;172(3):427-36. doi: 10.1111/cei.12065. Clin Exp Immunol. 2013. PMID: 23600831 Free PMC article.
-
Evaluating the Role of CXCR3 in Pain Modulation: A Literature Review.J Pain Res. 2020 Aug 6;13:1987-2001. doi: 10.2147/JPR.S254276. eCollection 2020. J Pain Res. 2020. PMID: 32821152 Free PMC article. Review.
-
'Neuroinflammation' differs categorically from inflammation: transcriptomes of Alzheimer's disease, Parkinson's disease, schizophrenia and inflammatory diseases compared.Neurogenetics. 2014 Aug;15(3):201-12. doi: 10.1007/s10048-014-0409-x. Epub 2014 Jun 15. Neurogenetics. 2014. PMID: 24928144
-
NF-κB signaling regulates cell-autonomous regulation of CXCL10 in breast cancer 4T1 cells.Exp Mol Med. 2017 Feb 17;49(2):e295. doi: 10.1038/emm.2016.148. Exp Mol Med. 2017. PMID: 28209986 Free PMC article.
References
-
- Rollins B. Chemokines. Blood. 1997;90:909–928. - PubMed
-
- Baggiolini M, Dewald B, Moser B. Human chemokines: an update. Annu Rev Immunol. 1997;15:675–705. - PubMed
-
- Schall TJ, Bacon KB. Chemokines, leukocyte trafficking, and inflammation. Curr Opin Immuno. 1994;6:865–873. - PubMed
-
- Hedrick J, Zlotnik A. Chemokines and lymphocyte biology. Curr Opin Immunol. 1996;8:343–347. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

