Lack of effect of tofacitinib (CP-690,550) on the pharmacokinetics of the CYP3A4 substrate midazolam in healthy volunteers: confirmation of in vitro data
- PMID: 22233204
- PMCID: PMC3394134
- DOI: 10.1111/j.1365-2125.2012.04168.x
Lack of effect of tofacitinib (CP-690,550) on the pharmacokinetics of the CYP3A4 substrate midazolam in healthy volunteers: confirmation of in vitro data
Abstract
What is already known about this subject: • Tofacitinib (CP-690,550) is a novel, oral Janus kinase inhibitor being investigated as a targeted immunomodulator and disease-modifying therapy in rheumatoid arthritis. • Non-renal elimination accounts for 70% of the total clearance of tofacitinib and the metabolism is primarily mediated by cytochrome P450 (CYP) 3A4. • This study was required to determine the effect of tofacitinib on the in vivo pharmacokinetics of a sensitive CYP3A4 substrate.
What this study adds: • The pharmacokinetics of midazolam, a sensitive CYP3A4 substrate, are not altered when co-administered with tofacitinib in healthy subjects. • Tofacitinib is unlikely to affect the clearance of drugs metabolized by CYP enzymes. • There is no need for dose adjustments of CYP substrates when co-administered with tofacitinib.
Aims: To investigate inhibitive and inductive effects of tofacitinib (CP-690,550), a Janus kinase inhibitor, on CYP3A4 function via in vitro and in vivo studies.
Methods: In vitro experiments were conducted to assess the inhibition and induction potential of tofacitinib for major drug metabolizing enzymes (CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6 and CYP3A4). A phase 1, randomized, open-label, two-way crossover study (NCT00902460) was conducted to confirm the lack of inhibitive/inductive effect on a sensitive CYP3A4 substrate, midazolam, in healthy subjects. Midazolam pharmacokinetics were assessed over 24 h following single dose 2 mg administration prior to administering tofacitinib and after twice daily dosing of tofacitinib 30 mg for 6 days. The primary endpoint was midazolam area under the concentration-time profile, from time 0 to infinity (AUC(0,∞)).
Results: In vitro studies demonstrated low potential for CYP inhibition (IC(50) estimates tofacitinib > 30 µm), CYP3A4 mRNA induction (observed at tofacitinib concentrations ≥ 25 µm) and no effect on enzymatic activity of CYP substrates. In the human study, AUC(0,∞) adjusted geometric mean ratio for midazolam plus tofacitinib to midazolam alone was 103.97% [90% confidence interval (CI) 95.57, 113.12], wholly within the pre-specified acceptance region (80, 125). The 90% CI for the ratio of adjusted geometric means of maximum plasma concentration (C(max) ) (95.98, 108.87) was also wholly within this acceptance region.
Conclusions: These data confirm a lack of an inhibitive or inductive effect of tofacitinib on CYP3A activity in humans and, in conjunction with in vitro data, support the conclusion that tofacitinib is unlikely to influence the CYP enzyme system as a whole.
© 2012 Pfizer Inc. British Journal of Clinical Pharmacology © 2012 The British Pharmacological Society.
Figures
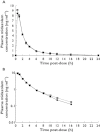
 ); Midazolam (2 mg) + tofacitinib (30 mg twice daily) (
); Midazolam (2 mg) + tofacitinib (30 mg twice daily) ( )
)
References
-
- Meyer DM, Jesson MI, Li X, Elrick MM, Funckes-Shippy CL, Warner JD, Gross CJ, Dowty ME, Ramaiah SK, Hirsch JL, Saabye MJ, Barks JL, Kishore N, Morris DL. Anti-inflammatory activity and neutrophil reductions mediated by the JAK1/JAK3 inhibitor, CP-690,550, in rat adjuvant-induced arthritis. J Inflamm. 2010;7:41. - PMC - PubMed
-
- Coombs JH, Bloom BJ, Breedveld FC, Fletcher MP, Gruben D, Kremer JM, Burgos-Vargas R, Wilkinson B, Zerbini CAF, Zwillich SH. Improved pain, physical functioning and health status in patients with rheumatoid arthritis treated with CP-690,550, an orally active Janus kinase (JAK) inhibitor: results from a randomised, double-blind, placebo-controlled trial. Ann Rheum Dis. 2010;69:413–6. - PubMed
-
- Kremer JM, Bloom BJ, Breedveld FC, Coombs JH, Fletcher MP, Gruben D, Krishnaswami S, Burgos-Vargas R, Wilkinson B, Zerbini CAF, Zwillich SH. The safety and efficacy of a JAK inhibitor in patients with active rheumatoid arthritis: results of a double-blind, placebo-controlled phase IIa trial of three dose levels of CP-690,550 versus placebo. Arthritis Rheum. 2009;60:1895–905. - PubMed
-
- Boy MG, Wang C, Wilkinson BE, Chow VF, Clucas AT, Krueger JG, Gaweco AS, Zwillich SH, Changelian PS, Chan G. Double-blind, placebo-controlled, dose-escalation study to evaluate the pharmacologic effect of CP-690,550 in patients with psoriasis. J Invest Dermatol. 2009;129:2299–302. - PubMed
-
- Busque S, Leventhal J, Brennan DC, Steinberg S, Klintmalm G, Shah T, Mulgaonkar S, Bromberg JS, Vincenti F, Hariharan S, Slakey D, Peddi VR, Fisher RA, Lawendy N, Wang C, Chan G. Calcineurin-inhibitor-free immunosuppression based on the JAK inhibitor CP-690,550: a pilot study in de novo kidney allograft recipients. Am J Transplant. 2009;9:1936–45. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

