Crizotinib (PF-02341066) reverses multidrug resistance in cancer cells by inhibiting the function of P-glycoprotein
- PMID: 22233293
- PMCID: PMC3419910
- DOI: 10.1111/j.1476-5381.2012.01849.x
Crizotinib (PF-02341066) reverses multidrug resistance in cancer cells by inhibiting the function of P-glycoprotein
Abstract
Background and purpose: Besides targeting the well-known oncogenic c-Met, crizotinib is the first oral tyrosine kinase inhibitor inhibiting anaplastic lymphoma kinase (ALK) in clinical trials for the treatment of non-small cell lung cancer. Here, we assessed the possible reversal of multidrug resistance (MDR) by crizotinib in vitro and in vivo.
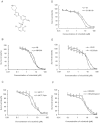
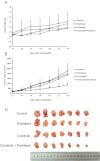
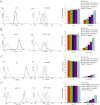
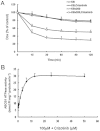
Experimental approach: 1-(4,5-Dimethylthiazol-2-yl)-3,5- diphenylformazan was used in vitro and xenografts in nude mice were used in vivo to investigate reversal of MDR by crizotinib. To understand the mechanisms for MDR reversal, the alterations of intracellular doxorubicin or rhodamine 123 accumulation, doxorubicin efflux, ABCB1 expression level, ATPase activity of ABCB1 and crizotinib-induced c-Met, Akt and ERK1/2 phosphorylation were examined.
Key results: Crizotinib significantly enhanced the cytotoxicity of chemotherapeutic agents which are also ABCB1 substrates, in MDR cells with no effect found on sensitive cells in vitro and in vivo. Additionally, crizotinib significantly increased intracellular accumulation of rhodamine 123 and doxorubicin and inhibited the drug efflux in ABCB1-overexpressing MDR cells. Further studies showed that crizotinib enhanced the ATPase activity of ABCB1 in a concentration-dependent manner. However, expression of ABCB1 was not affected, and reversal of MDR by crizotinib was not related to the phosphorylation of c-Met, Akt or ERK1/2. Importantly, crizotinib significantly enhanced the effect of paclitaxel against KBv200 cell xenografts in nude mice.
Conclusions and implications: Crizotinib reversed ABCB1-mediated MDR by inhibiting ABCB1 transport function without affecting ABCB1 expression or blocking the Akt or ERK1/2 pathways. These findings are useful for planning combination chemotherapy of crizotinib with conventional chemotherapeutic drugs.
© 2012 The Authors. British Journal of Pharmacology © 2012 The British Pharmacological Society.
Figures


References
-
- Ambudkar SV, Dey S, Hrycyna CA, Ramachandra M, Pastan I, Gottesman MM. Biochemical, cellular, and pharmacological aspects of the multidrug transporter. Annu Rev Pharmacol Toxicol. 1999;39:361–398. - PubMed
-
- Azam M, Latek RR, Daley GQ. Mechanisms of autoinhibition and STI-571/imatinib resistance revealed by mutagenesis of BCR-ABL. Cell. 2003;112:831–843. - PubMed
-
- Bakos E, Homolya L. Portrait of multifaceted transporter, the multidrug resistance-associated protein 1 (MRP1/ABCC1) Pflugers Arch. 2007;453:621–641. - PubMed
-
- Blume-Jensen P, Hunter T. Oncogenic kinase signalling. Nature. 2001;411:355–365. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

