Protein kinase D2 has a restricted but critical role in T-cell antigen receptor signalling in mature T-cells
- PMID: 22233340
- PMCID: PMC3462612
- DOI: 10.1042/BJ20111700
Protein kinase D2 has a restricted but critical role in T-cell antigen receptor signalling in mature T-cells
Abstract
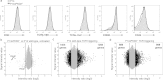
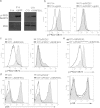
PKD (protein kinase D) 2 is a serine/threonine kinase activated by diacylglycerol in response to engagement of antigen receptors in lymphocytes. To explore PKD2 regulation and function in TCR (T-cell antigen receptor) signal transduction we expressed TCR complexes with fixed affinity for self antigens in the T-cells of PKD2-null mice or mice deficient in PKD2 catalytic activity. We also developed a single cell assay to quantify PKD2 activation as T-cells respond to developmental stimuli or engagement of α/β TCR complexes in vivo. Strikingly, PKD2 loss caused increases in thymic output, lymphadenopathy and splenomegaly in TCR transgenic mice. The precise magnitude and timing of PKD2 activation during T-cell development is thus critical to regulate thymic homoeostasis. PKD2-null T-cells that exit the thymus have a normal transcriptome, but show a limited and abnormal transcriptional response to antigen. Transcriptional profiling reveals the full consequences of PKD2 loss and maps in detail the selective, but critical, function for PKD2 in signalling by α/β mature TCR complexes in peripheral T-cells.
Figures



References
-
- Salmond R. J., Filby A., Qureshi I., Caserta S., Zamoyska R. T-cell receptor proximal signaling via the Src-family kinases, Lck and Fyn, influences T-cell activation, differentiation, and tolerance. Immunol. Rev. 2009;228:9–22. - PubMed
-
- Olenchock B. A., Guo R., Carpenter J. H., Jordan M., Topham M. K., Koretzky G. A., Zhong X. P. Disruption of diacylglycerol metabolism impairs the induction of T cell anergy. Nat. Immunol. 2006;7:1174–1181. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

