Inhibition of T-type Ca²⁺ channels by endostatin attenuates human glioblastoma cell proliferation and migration
- PMID: 22233416
- PMCID: PMC3417444
- DOI: 10.1111/j.1476-5381.2012.01852.x
Inhibition of T-type Ca²⁺ channels by endostatin attenuates human glioblastoma cell proliferation and migration
Abstract
Background and purpose: Endostatin (ES) is a c-terminal proteolytic fragment of collagen XVIII with promising antitumour properties in several tumour models, including human glioblastoma. We hypothesized that this peptide could interact with plasma membrane ion channels and modulate their functions.
Experimental approach: Using cell proliferation and migration assays, patch clamp and Western blot analysis, we studied the effects of ES on the proliferation and migration of human glioblastoma U87 cells, mediated by T-type Ca²⁺ channels.
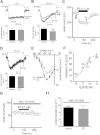
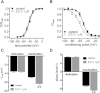
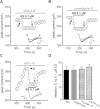
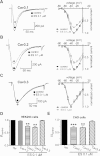
Key results: Extracellular application of ES reversibly inhibited T-type Ca²⁺ channel currents (T-currents) in U87 cells, whereas L-type Ca²⁺ currents were not affected. This inhibitory effect was associated with a hyperpolarizing shift in the voltage-dependence of inactivation but was independent of G-protein and protein tyrosine kinase-mediated pathways. All three α₁ subunits of T-type Ca²⁺ channels (Ca(V) 3), α(1G) (Ca(V) 3.1), α(1H) (Ca(V) 3.2) and α(1I) (Ca(V) 3.3), were endogenously expressed in U87 cells. Using transfected HEK293 or CHO cells, we showed that only Ca(V) 3.1 and Ca(V) 3.2, but not Ca(V) 3.3 or Ca(V) 1.2 (L-type), channel currents were significantly inhibited. More interestingly, ES inhibited the proliferation and migration of U87 cells in a dose-dependent manner. Pretreatment of the cells with the specific T-type Ca²⁺ channel blocker mibefradil occluded these inhibitory effects of ES.
Conclusion and implications: This study provides the first evidence that the antitumour effects of ES on glioblastoma cells is through direct inhibition of T-type Ca²⁺ channels and gives new insights into the future development of a new class of antiglioblastoma agents that target the proliferation and migration of these cells.
© 2012 The Authors. British Journal of Pharmacology © 2012 The British Pharmacological Society.
Figures

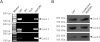

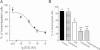
Comment in
-
Functional role of T-type calcium channels in tumour growth and progression: prospective in cancer therapy.Br J Pharmacol. 2012 Jun;166(4):1244-6. doi: 10.1111/j.1476-5381.2012.01908.x. Br J Pharmacol. 2012. PMID: 22352795 Free PMC article.
Similar articles
-
Functional role of T-type calcium channels in tumour growth and progression: prospective in cancer therapy.Br J Pharmacol. 2012 Jun;166(4):1244-6. doi: 10.1111/j.1476-5381.2012.01908.x. Br J Pharmacol. 2012. PMID: 22352795 Free PMC article.
-
Involvement of CaV3.1 T-type calcium channels in cell proliferation in mouse preadipocytes.Am J Physiol Cell Physiol. 2010 Jun;298(6):C1414-23. doi: 10.1152/ajpcell.00488.2009. Epub 2010 Mar 24. Am J Physiol Cell Physiol. 2010. PMID: 20457833
-
Endostatin inhibits T-type Ca2+ channel current in guinea pig ventricular myocyte.J Vet Med Sci. 2015 Oct;77(10):1289-91. doi: 10.1292/jvms.14-0551. Epub 2015 May 4. J Vet Med Sci. 2015. PMID: 25947888 Free PMC article.
-
Low-voltage-activated T-type Ca2+ channel inhibitors as new tools in the treatment of glioblastoma: the role of endostatin.Pflugers Arch. 2014 Apr;466(4):811-8. doi: 10.1007/s00424-013-1427-5. Epub 2014 Jan 10. Pflugers Arch. 2014. PMID: 24407946 Review.
-
Voltage-dependent calcium channels.Gen Physiol Biophys. 2005 Jun;24 Suppl 1:1-78. Gen Physiol Biophys. 2005. PMID: 16096350 Review.
Cited by
-
Calcium channel expression and applicability as targeted therapies in melanoma.Biomed Res Int. 2015;2015:587135. doi: 10.1155/2015/587135. Epub 2015 Feb 1. Biomed Res Int. 2015. PMID: 25710007 Free PMC article. Review.
-
Effects of BKCa and Kir2.1 Channels on Cell Cycling Progression and Migration in Human Cardiac c-kit+ Progenitor Cells.PLoS One. 2015 Sep 21;10(9):e0138581. doi: 10.1371/journal.pone.0138581. eCollection 2015. PLoS One. 2015. PMID: 26390131 Free PMC article.
-
Ion Channels in Glioma Malignancy.Rev Physiol Biochem Pharmacol. 2021;181:223-267. doi: 10.1007/112_2020_44. Rev Physiol Biochem Pharmacol. 2021. PMID: 32930879 Review.
-
Contribution of S4 segments and S4-S5 linkers to the low-voltage activation properties of T-type CaV3.3 channels.PLoS One. 2018 Feb 23;13(2):e0193490. doi: 10.1371/journal.pone.0193490. eCollection 2018. PLoS One. 2018. PMID: 29474447 Free PMC article.
-
Heterogeneity of intracellular calcium signaling of glioblastoma cells depends on intratumoral location and migration state.Neurooncol Adv. 2025 Mar 5;7(1):vdaf055. doi: 10.1093/noajnl/vdaf055. eCollection 2025 Jan-Dec. Neurooncol Adv. 2025. PMID: 40264943 Free PMC article.
References
-
- Adams PJ, Snutch TP. Calcium channelopathies: voltage-gated calcium channels. Subcell Biochem. 2007;45:215–251. - PubMed
-
- Boehm T, Folkman J, Browder T, O'Reilly MS. Antiangiogenic therapy of experimental cancer does not induce acquired drug resistance. Nature. 1997;390:404–407. - PubMed
-
- Cao Y. Endogenous angiogenesis inhibitors and their therapeutic implications. Int J Biochem Cell Biol. 2001;33:357–369. - PubMed
-
- Chemin J, Monteil A, Briquaire C, Richard S, Perez-Reyes E, Nargeot J, et al. Overexpression of T-type calcium channels in HEK-293 cells increases intracellular calcium without affecting cellular proliferation. FEBS Lett. 2000;478:166–172. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

