Enzymatic activities of isolated cytochrome bc₁-like complexes containing fused cytochrome b subunits with asymmetrically inactivated segments of electron transfer chains
- PMID: 22233445
- PMCID: PMC3269193
- DOI: 10.1021/bi2016316
Enzymatic activities of isolated cytochrome bc₁-like complexes containing fused cytochrome b subunits with asymmetrically inactivated segments of electron transfer chains
Abstract
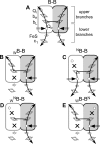
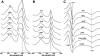
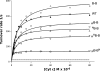
Homodimeric structure of cytochrome bc₁, a common component of biological energy conversion systems, builds in four catalytic quinone oxidation/reduction sites and four chains of cofactors (branches) that, connected by a centrally located bridge, form a symmetric H-shaped electron transfer system. The mechanism of operation of this complex system is under constant debate. Here, we report on isolation and enzymatic examination of cytochrome bc₁-like complexes containing fused cytochrome b subunits in which asymmetrically introduced mutations inactivated individual branches in various combinations. The structural asymmetry of those forms was confirmed spectroscopically. All the asymmetric forms corresponding to cytochrome bc₁ with partial or full inactivation of one monomer retain high enzymatic activity but at the same time show a decrease in the maximum turnover rate by a factor close to 2. This strongly supports the model assuming independent operation of monomers. The cross-inactivated form corresponding to cytochrome bc₁ with disabled complementary parts of each monomer retains the enzymatic activity at the level that, for the first time on isolated from membranes and purified to homogeneity preparations, demonstrates that intermonomer electron transfer through the bridge effectively sustains the enzymatic turnover. The results fully support the concept that electrons freely distribute between the four catalytic sites of a dimer and that any path connecting the catalytic sites on the opposite sides of the membrane is enzymatically competent. The possibility to examine enzymatic properties of isolated forms of asymmetric complexes constructed using the cytochrome b fusion system extends the array of tools available for investigating the engineering of dimeric cytochrome bc₁ from the mechanistic and physiological perspectives.
Figures

Similar articles
-
An electronic bus bar lies in the core of cytochrome bc1.Science. 2010 Jul 23;329(5990):451-4. doi: 10.1126/science.1190899. Science. 2010. PMID: 20651150 Free PMC article.
-
Fusing two cytochromes b of Rhodobacter capsulatus cytochrome bc1 using various linkers defines a set of protein templates for asymmetric mutagenesis.Protein Eng Des Sel. 2012 Jan;25(1):15-25. doi: 10.1093/protein/gzr055. Epub 2011 Nov 25. Protein Eng Des Sel. 2012. PMID: 22119789 Free PMC article.
-
Hybrid fusions show that inter-monomer electron transfer robustly supports cytochrome bc1 function in vivo.Biochem Biophys Res Commun. 2014 Aug 22;451(2):270-5. doi: 10.1016/j.bbrc.2014.07.117. Epub 2014 Aug 1. Biochem Biophys Res Commun. 2014. PMID: 25089001 Free PMC article.
-
Molecular mechanisms of superoxide production by complex III: a bacterial versus human mitochondrial comparative case study.Biochim Biophys Acta. 2013 Nov-Dec;1827(11-12):1332-9. doi: 10.1016/j.bbabio.2013.03.009. Epub 2013 Mar 28. Biochim Biophys Acta. 2013. PMID: 23542447 Free PMC article. Review.
-
Activated Q-cycle as a common mechanism for cytochrome bc1 and cytochrome b6f complexes.Biochim Biophys Acta. 2010 Dec;1797(12):1858-68. doi: 10.1016/j.bbabio.2010.07.008. Epub 2010 Jul 25. Biochim Biophys Acta. 2010. PMID: 20650262 Review.
Cited by
-
A robust genetic system for producing heterodimeric native and mutant cytochrome bc(1).Biochemistry. 2013 Oct 15;52(41):7184-95. doi: 10.1021/bi400560p. Epub 2013 Oct 1. Biochemistry. 2013. PMID: 24028512 Free PMC article.
-
pH and Potential Transients of the bc1 Complex Co-Reconstituted in Proteo-Lipobeads with the Reaction Center from Rb. sphaeroides.J Phys Chem B. 2017 Jan 12;121(1):143-152. doi: 10.1021/acs.jpcb.6b11116. Epub 2017 Jan 4. J Phys Chem B. 2017. PMID: 27992230 Free PMC article.
-
Distinct properties of semiquinone species detected at the ubiquinol oxidation Qo site of cytochrome bc1 and their mechanistic implications.J R Soc Interface. 2016 May;13(118):20160133. doi: 10.1098/rsif.2016.0133. J R Soc Interface. 2016. PMID: 27194483 Free PMC article. Review.
-
The High-Spin Heme b L Mutant Exposes Dominant Reaction Leading to the Formation of the Semiquinone Spin-Coupled to the [2Fe-2S]+ Cluster at the Qo Site of Rhodobacter capsulatus Cytochrome bc 1.Front Chem. 2021 May 7;9:658877. doi: 10.3389/fchem.2021.658877. eCollection 2021. Front Chem. 2021. PMID: 34026724 Free PMC article.
-
Low-cost stopped-flow and freeze-quench device for double mixing.HardwareX. 2023 Mar 1;14:e00409. doi: 10.1016/j.ohx.2023.e00409. eCollection 2023 Jun. HardwareX. 2023. PMID: 36910022 Free PMC article.
References
-
- Kramer D. M., Nitschke W., and Cooley J. W. (2009) The cytochrome bc1 and related bc complexes: the Rieske/cytochrome b complex as the functional core of a central electron/proton transfer complex, in The Purple Phototrophic Bacteria (Hunter N., Daldal F., Thurnauer M. C., and Beatty J. T., Eds.) Springer, The Netherlands.
-
- Crofts A. R.; Holland J. T.; Victoria D.; Kolling D. R. J.; Dikanov S. A.; Gilbreth R.; Lhee S.; Kuras R.; Guergova Kuras M. (2008) The Q-cycle reviewed: how well does a monomeric mechanism of the bc1 complex account for the function of a dimeric complex?. Biochim. Biophys. Acta 1777, 1001–1019. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

