An obligatory role for lung infiltrating B cells in the immunopathogenesis of obliterative airway disease induced by antibodies to MHC class I molecules
- PMID: 22233464
- PMCID: PMC3721353
- DOI: 10.1111/j.1600-6143.2011.03917.x
An obligatory role for lung infiltrating B cells in the immunopathogenesis of obliterative airway disease induced by antibodies to MHC class I molecules
Abstract
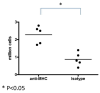
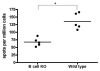
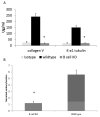
Using a murine model, we demonstrated that endobronchial administration of antibodies (Abs) to major histocompatibility complex (MHC) class I results in cellular infiltration, epithelial metaplasia, fibrosis and obstruction of the small airways (obliterative airway disease [OAD]) mediated predominantly by Th17 responses to self-antigens. This resembles bronchiolitis obliterans syndrome developed following human lung transplantation. Since B cells play a crucial role in induction of autoimmune responses, we defined the role of B cells and its antigen presenting properties in induction of OAD in this study. Anti-MHC class I was administered endobronchially in B(-/-) and wild-type mice. In contrast to wild type, B(-/-) animals did not demonstrate cellular infiltration, epithelial metaplasia and obstruction of airways following anti-MHC. Frequency of K-α1 tubulin and CollagenV-specific IL-17 cells was significantly decreased in B(-/-) mice. As expected, Abs against self-antigens and germinal center formation were not developed in B(-/-) mice. Thus, we conclude that B cells and its antigen presenting capacity play an important role in induction of immune responses to self-antigens and immunopathogenesis of OAD following the administration of anti-MHC. Therefore, strategies to block B-cell and its antigen presenting functions should be considered for preventing the development of chronic rejection.
© Copyright 2012 The American Society of Transplantation and the American Society of Transplant Surgeons.
Figures
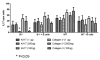






References
-
- Lange W. Uber eine eigenthumbiche erkankung der kleinen broncheim and bronchiolen. Deutsche Arch Klin Med. 1901;70:342–364.
-
- Sundaresan RS, Trulock EP, Mohanakumar T, Cooper JD, Patterson GA. Prevalence and outcome of bronchiolitis obliterans syndrome after lung transplantation. Washington University Lung Transplant Group. Annals of Thoracic Surgery. 1995;60:1341–1347. - PubMed
-
- Valentine VG, Robbins RC, Berry GJ, Patel HR, Reichenspurner H, Reitz BA, et al. Actuarial survival of heart-lung and bilateral sequential lung transplant recipients with obliterative bronchiolitis. J Heart Lung Transplant. 1996;15:371–383. - PubMed
-
- Smith MA, Sundaresan S, Mohanakumar T, Trulock EP, Lynch JP, Phelan DL, et al. Effect of development of antibodies to HLA and cytomegalovirus mismatch on lung transplantation survival and development of bronchiolitis obliterans syndrome. J Thorac Cardiovasc Surg. 1998;116(5):812–820. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

