Toll-like receptor 4 contributes to blood pressure regulation and vascular contraction in spontaneously hypertensive rats
- PMID: 22233532
- PMCID: PMC4004345
- DOI: 10.1042/CS20110523
Toll-like receptor 4 contributes to blood pressure regulation and vascular contraction in spontaneously hypertensive rats
Abstract
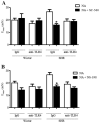
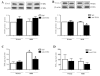
Activation of TLRs (Toll-like receptors) induces gene expression of proteins involved in the immune system response. TLR4 has been implicated in the development and progression of CVDs (cardio-vascular diseases). Innate and adaptive immunity contribute to hypertension-associated end-organ damage, although the mechanism by which this occurs remains unclear. In the present study, we hypothesize that inhibition of TLR4 decreases BP (blood pressure) and improves vascular contractility in resistance arteries from SHR (spontaneously hypertensive rats). TLR4 protein expression in mesenteric resistance arteries was higher in 15-week-old SHR than in age-matched Wistar controls or in 5-week-old SHR. To decrease the activation of TLR4, 15-week-old SHR and Wistar rats were treated with anti-TLR4 (anti-TLR4 antibody) or non-specific IgG control antibody for 15 days (1 μg per day, intraperitoneal). Treatment with anti-TLR4 decreased MAP (mean arterial pressure) as well as TLR4 protein expression in mesenteric resistance arteries and IL-6 (interleukin 6) serum levels from SHR when compared with SHR treated with IgG. No changes in these parameters were found in treated Wistar control rats. Mesenteric resistance arteries from anti-TLR4-treated SHR exhibited decreased maximal contractile response to NA (noradrenaline) compared with IgG-treated SHR. Inhibition of COX (cyclo-oxygenase)-1 and COX-2, enzymes related to inflammatory pathways, decreased NA responses only in mesenteric resistance arteries of SHR treated with IgG. COX-2 expression and TXA2 (thromboxane A2) release were decreased in SHR treated with anti-TLR4 compared with IgG-treated SHR. Our results suggest that TLR4 activation contributes to increased BP, low-grade inflammation and plays a role in the augmented vascular contractility displayed by SHR.
Figures

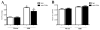

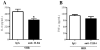
References
-
- Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. - PubMed
-
- Kawai T, Akira S. TLR signaling. Cell Death Differ. 2006;13:816–825. - PubMed
-
- Frantz S, Ertl G, Bauersachs J. Mechanisms of disease: Toll-like receptors in cardiovascular disease. Nat Clin Pract Cardiovasc Med. 2007;4:444–454. - PubMed
-
- Pasterkamp G, Van Keulen JK, De Kleijn DP. Role of Toll-like receptor 4 in the initiation and progression of atherosclerotic disease. Eur J Clin Invest. 2004;34:328–334. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

