Synaptic neurotransmitter-gated receptors
- PMID: 22233560
- PMCID: PMC3282413
- DOI: 10.1101/cshperspect.a009662
Synaptic neurotransmitter-gated receptors
Abstract
Since the discovery of the major excitatory and inhibitory neurotransmitters and their receptors in the brain, many have deliberated over their likely structures and how these may relate to function. This was initially satisfied by the determination of the first amino acid sequences of the Cys-loop receptors that recognized acetylcholine, serotonin, GABA, and glycine, followed later by similar determinations for the glutamate receptors, comprising non-NMDA and NMDA subtypes. The last decade has seen a rapid advance resulting in the first structures of Cys-loop receptors, related bacterial and molluscan homologs, and glutamate receptors, determined down to atomic resolution. This now provides a basis for determining not just the complete structures of these important receptor classes, but also for understanding how various domains and residues interact during agonist binding, receptor activation, and channel opening, including allosteric modulation. This article reviews our current understanding of these mechanisms for the Cys-loop and glutamate receptor families.
Figures
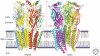





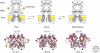

References
-
- Absalom NL, Lewis TM, Kaplan W, Pierce KD, Schofield PR 2003. Role of charged residues in coupling ligand binding and channel activation in the extracellular domain of the glycine receptor. J Biol Chem 278: 50151–50157 - PubMed
-
- Amin J, Weiss DS 1996. Insights into the activation mechanism of ρ1 GABA receptors obtained by coexpression of wild type and activation-impaired subunits. Proc R Soc Lond B Biol Sci 263: 273–282 - PubMed
-
- Araud T, Wonnacott S, Bertrand D 2010. Associated proteins: The universal toolbox controlling ligand gated ion channel function. Biochem Pharmacol 80: 160–169 - PubMed
-
- Arevalo E, Chiara DC, Forman SA, Cohen JB, Miller KW 2005. Gating-enhanced accessibility of hydrophobic sites within the transmembrane region of the nicotinic acetylcholine receptor’s δ-subunit. A time-resolved photolabeling study. J Biol Chem 280: 13631–13640 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
