Programmable multivalent display of receptor ligands using peptide nucleic acid nanoscaffolds
- PMID: 22233624
- PMCID: PMC3518395
- DOI: 10.1038/ncomms1629
Programmable multivalent display of receptor ligands using peptide nucleic acid nanoscaffolds
Abstract
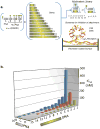
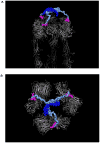
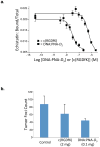
Multivalent effects dictate the binding affinity of multiple ligands on one molecular entity to receptors. Integrins are receptors that mediate cell attachment through multivalent binding to peptide sequences within the extracellular matrix, and overexpression promotes the metastasis of some cancers. Multivalent display of integrin antagonists enhances their efficacy, but current scaffolds have limited ranges and precision for the display of ligands. Here we present an approach to studying multivalent effects across wide ranges of ligand number, density, and three-dimensional arrangement. Using L-lysine γ-substituted peptide nucleic acids, the multivalent effects of an integrin antagonist were examined over a range of 1-45 ligands. The optimal construct improves the inhibitory activity of the antagonist by two orders of magnitude against the binding of melanoma cells to the extracellular matrix in both in vitro and in vivo models.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



Similar articles
-
Programmable nanoscaffolds that control ligand display to a G-protein-coupled receptor in membranes to allow dissection of multivalent effects.J Am Chem Soc. 2014 Sep 3;136(35):12296-303. doi: 10.1021/ja504288s. Epub 2014 Aug 25. J Am Chem Soc. 2014. PMID: 25116377 Free PMC article.
-
DNA display of PNA-tagged ligands: a versatile strategy to screen libraries and control geometry of multidentate ligands.Artif DNA PNA XNA. 2012 Jul 1;3(3):105-8. doi: 10.4161/adna.21108. Epub 2012 Jul 1. Artif DNA PNA XNA. 2012. PMID: 22871882 Free PMC article.
-
[Molecular mechanisms of integrin-ligand interaction].Tanpakushitsu Kakusan Koso. 1997 Jul;42(10 Suppl):1679-86. Tanpakushitsu Kakusan Koso. 1997. PMID: 9279099 Review. Japanese. No abstract available.
-
Cell adhesion and motility depend on nanoscale RGD clustering.J Cell Sci. 2000 May;113 ( Pt 10):1677-86. doi: 10.1242/jcs.113.10.1677. J Cell Sci. 2000. PMID: 10769199
-
In Situ Detection of Integrin Ligands.Curr Protoc Cell Biol. 2014 Dec 11;65:9.7.1-17. doi: 10.1002/0471143030.cb0907s65. Curr Protoc Cell Biol. 2014. PMID: 26061156 Review.
Cited by
-
Visible Light-Induced Templated Metathesis of Peptide-Nucleic Acid Conjugates with a Diselenide Bridge.Biomolecules. 2023 Nov 20;13(11):1676. doi: 10.3390/biom13111676. Biomolecules. 2023. PMID: 38002358 Free PMC article.
-
Design of peptide affinity ligands for S-protein: a comparison of combinatorial and de novo design strategies.Mol Divers. 2013 May;17(2):357-69. doi: 10.1007/s11030-013-9436-z. Epub 2013 Mar 27. Mol Divers. 2013. PMID: 23532725
-
Lighting Up the Force: Investigating Mechanisms of Mechanotransduction Using Fluorescent Tension Probes.Mol Cell Biol. 2015 Aug;35(15):2570-82. doi: 10.1128/MCB.00195-15. Epub 2015 Jun 1. Mol Cell Biol. 2015. PMID: 26031334 Free PMC article. Review.
-
Androgen receptor antagonism by divalent ethisterone conjugates in castrate-resistant prostate cancer cells.ACS Chem Biol. 2012 Oct 19;7(10):1693-701. doi: 10.1021/cb300332w. Epub 2012 Aug 7. ACS Chem Biol. 2012. PMID: 22871957 Free PMC article.
-
Green fluorescent protein nanopolygons as monodisperse supramolecular assemblies of functional proteins with defined valency.Nat Commun. 2015 May 14;6:7134. doi: 10.1038/ncomms8134. Nat Commun. 2015. PMID: 25972078 Free PMC article.
References
-
- Nemeth JA, et al. Aplha-v Integrins as Therapeutic Targets in Oncology. Cancer Invest. 2007;25:632–646. - PubMed
-
- Auzzas L, et al. Targeting αvβ3 integrin: design and applications of mono-and multifunctional RGD-based peptides and semipeptides. Curr Med Chem. 2010;17:1255–1299. - PubMed
-
- Stupp R, et al. Phase I/IIa Study of Cilengitide and Temozolomide With Concomitant Radiotherapy Followed by Cilengitide and Temozolomide Maintenance Therapy in Patients With Newly Diagnosed Glioblastoma. J Clin Oncol. 2010;28:2712–2718. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

