Programmable multivalent display of receptor ligands using peptide nucleic acid nanoscaffolds
- PMID: 22233624
- PMCID: PMC3518395
- DOI: 10.1038/ncomms1629
Programmable multivalent display of receptor ligands using peptide nucleic acid nanoscaffolds
Abstract
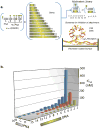
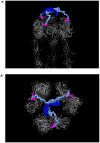
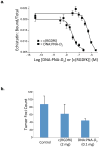
Multivalent effects dictate the binding affinity of multiple ligands on one molecular entity to receptors. Integrins are receptors that mediate cell attachment through multivalent binding to peptide sequences within the extracellular matrix, and overexpression promotes the metastasis of some cancers. Multivalent display of integrin antagonists enhances their efficacy, but current scaffolds have limited ranges and precision for the display of ligands. Here we present an approach to studying multivalent effects across wide ranges of ligand number, density, and three-dimensional arrangement. Using L-lysine γ-substituted peptide nucleic acids, the multivalent effects of an integrin antagonist were examined over a range of 1-45 ligands. The optimal construct improves the inhibitory activity of the antagonist by two orders of magnitude against the binding of melanoma cells to the extracellular matrix in both in vitro and in vivo models.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



References
-
- Nemeth JA, et al. Aplha-v Integrins as Therapeutic Targets in Oncology. Cancer Invest. 2007;25:632–646. - PubMed
-
- Auzzas L, et al. Targeting αvβ3 integrin: design and applications of mono-and multifunctional RGD-based peptides and semipeptides. Curr Med Chem. 2010;17:1255–1299. - PubMed
-
- Stupp R, et al. Phase I/IIa Study of Cilengitide and Temozolomide With Concomitant Radiotherapy Followed by Cilengitide and Temozolomide Maintenance Therapy in Patients With Newly Diagnosed Glioblastoma. J Clin Oncol. 2010;28:2712–2718. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

