Chemical data quantify Deepwater Horizon hydrocarbon flow rate and environmental distribution
- PMID: 22233807
- PMCID: PMC3528560
- DOI: 10.1073/pnas.1110564109
Chemical data quantify Deepwater Horizon hydrocarbon flow rate and environmental distribution
Abstract
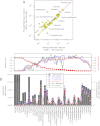
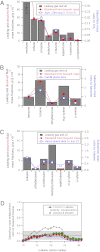
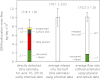
Detailed airborne, surface, and subsurface chemical measurements, primarily obtained in May and June 2010, are used to quantify initial hydrocarbon compositions along different transport pathways (i.e., in deep subsurface plumes, in the initial surface slick, and in the atmosphere) during the Deepwater Horizon oil spill. Atmospheric measurements are consistent with a limited area of surfacing oil, with implications for leaked hydrocarbon mass transport and oil drop size distributions. The chemical data further suggest relatively little variation in leaking hydrocarbon composition over time. Although readily soluble hydrocarbons made up ∼25% of the leaking mixture by mass, subsurface chemical data show these compounds made up ∼69% of the deep plume mass; only ∼31% of the deep plume mass was initially transported in the form of trapped oil droplets. Mass flows along individual transport pathways are also derived from atmospheric and subsurface chemical data. Subsurface hydrocarbon composition, dissolved oxygen, and dispersant data are used to assess release of hydrocarbons from the leaking well. We use the chemical measurements to estimate that (7.8 ± 1.9) × 10(6) kg of hydrocarbons leaked on June 10, 2010, directly accounting for roughly three-quarters of the total leaked mass on that day. The average environmental release rate of (10.1 ± 2.0) × 10(6) kg/d derived using atmospheric and subsurface chemical data agrees within uncertainties with the official average leak rate of (10.2 ± 1.0) × 10(6) kg/d derived using physical and optical methods.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- McNutt MK, et al. Assessment of Flow Rate Estimates for the Deepwater Horizon/Macondo Well Oil Spill. Flow Rate Technical Group Report to the National Incident Command Interagency Solutions Group. 2011. Available at http://www.doi.gov/deepwaterhorizon/loader.cfm?csModule=security/getfile.... Accessed December 13, 2011.
-
- Ryerson TB, et al. Atmospheric emissions from the Deepwater Horizon spill constrain air–water partitioning, hydrocarbon fate, and leak rate. Geophys Res Lett. 2011;38(L07803) 10.1029/2011GL0467.
-
- Graham R, et al. Deep Water: The Gulf Oil Disaster and the Future of Offshore Drilling. 2010. National Commission on the BP Deepwater Horizon Oil Spill and Offshore Drilling Report to the President. Available at http://www.gpoaccess.gov/deepwater/deepwater.pdf. Accessed December 13, 2011.
-
- de Gouw JA, et al. Organic aerosol formation downwind from the Deepwater Horizon oil spill. Science. 2011;331:1295–1299. - PubMed

