Oral nutritional supplements containing n-3 polyunsaturated fatty acids affect quality of life and functional status in lung cancer patients during multimodality treatment: an RCT
- PMID: 22234041
- PMCID: PMC3303137
- DOI: 10.1038/ejcn.2011.214
Oral nutritional supplements containing n-3 polyunsaturated fatty acids affect quality of life and functional status in lung cancer patients during multimodality treatment: an RCT
Abstract
Background/objectives: Our objective was to investigate effects of an oral nutritional supplement containing n-3 polyunsaturated fatty acids (FAs) on quality of life, performance status, handgrip strength and physical activity in patients with non-small cell lung cancer (NSCLC) undergoing multimodality treatment.
Subjects/methods: In a double-blind experiment, 40 patients with stage III NSCLC were randomised to receive 2 cans/day of a protein- and energy-dense oral nutritional supplement containing n-3 polyunsaturated FAs (2.02 g eicosapentaenoic acid+0.92 g docosahexaenoic acid/day) or an isocaloric control supplement, during multimodality treatment. Quality of life, Karnofsky Performance Status, handgrip strength and physical activity (by wearing an accelerometer) were assessed. Effects of intervention were analysed by generalised estimating equations. P-values <0.05 were regarded as statistically significant.
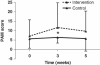
Results: The intervention group reported significantly higher on the quality of life parameters, physical and cognitive function (B=11.6 and B=20.7, P<0.01), global health status (B=12.2, P=0.04) and social function (B=22.1, P=0.04) than the control group after 5 weeks. The intervention group showed a higher Karnofsky Performance Status (B=5.3, P=0.04) than the control group after 3 weeks. Handgrip strength did not significantly differ between groups over time. The intervention group tended to have a higher physical activity than the control group after 3 and 5 weeks (B=6.6, P=0.04 and B=2.5, P=0.05).
Conclusion: n-3 Polyunsaturated FAs may beneficially affect quality of life, performance status and physical activity in patients with NSCLC undergoing multimodality treatment.
Figures
References
-
- Mountain CF. A new international staging system for lung cancer. Chest. 1986;89:225S–233S. - PubMed
-
- Price KA, Azzoli CG, Gaspar LE. Chemoradiation for unresectable stage III non-small cell lung cancer. Semin Thorac Cardiovasc Surg. 2008;20:204–209. - PubMed
-
- De Leyn P, Vansteenkiste J, Lievens Y, Van RD, Nafteux P, Decker G, et al. Survival after trimodality treatment for superior sulcus and central T4 non-small cell lung cancer. J Thorac Oncol. 2009;4:62–68. - PubMed
-
- van Meerbeeck JP, Kramer GW, Van Schil PE, Legrand C, Smit EF, Schramel F, et al. Randomized controlled trial of resection versus radiotherapy after induction chemotherapy in stage IIIA-N2 non-small-cell lung cancer. J Natl Cancer Inst. 2007;99:442–450. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

