Silica-based nanoprobes for biomedical imaging and theranostic applications
- PMID: 22234515
- PMCID: PMC3777230
- DOI: 10.1039/c2cs15229k
Silica-based nanoprobes for biomedical imaging and theranostic applications
Abstract
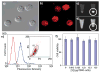
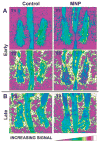
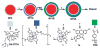
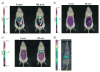
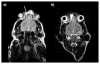
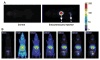
Nanoparticle-based contrast agents are attracting a great deal of attention for various biomedical imaging and theranostic applications. Compared to conventional contrast agents, nanoparticles possess several potential advantages to improve in vivo detection and to enhance targeting efficiency. Silica-based nanoprobes can be engineered to achieve longer blood circulation times, specific clearance pathways, and multivalent binding. In this tutorial review, we summarize the latest progress on designing silica-based nanoprobes for imaging and theranostic applications. The synthesis of both solid silica and mesoporous silica nanoparticles is described, along with different approaches used for surface functionalization. Special emphasis is placed on the application of silica-based nanoprobes in optical, magnetic resonance, and multimodal imaging. The latest breakthroughs in the applications of silica nanoparticles as theranostic agents are also highlighted.
Figures









References
-
- Huang X, Jain PK, El-Sayed IH, El-Sayed MA. Nanomedicine. 2007;2:681–693. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

