Wnt/β-catenin signaling and AXIN1 regulate apoptosis triggered by inhibition of the mutant kinase BRAFV600E in human melanoma
- PMID: 22234612
- PMCID: PMC3297477
- DOI: 10.1126/scisignal.2002274
Wnt/β-catenin signaling and AXIN1 regulate apoptosis triggered by inhibition of the mutant kinase BRAFV600E in human melanoma
Abstract
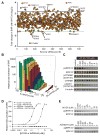
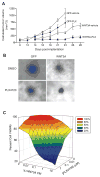
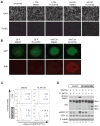
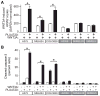
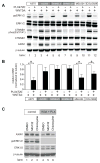
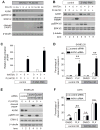
Because the Wnt/β-catenin signaling pathway is linked to melanoma pathogenesis and to patient survival, we conducted a kinome small interfering RNA (siRNA) screen in melanoma cells to expand our understanding of the kinases that regulate this pathway. We found that BRAF signaling, which is constitutively activated in many melanomas by the BRAF(V600E) mutation, inhibits Wnt/β-catenin signaling in human melanoma cells. Because inhibitors of BRAF(V600E) show promise in ongoing clinical trials, we investigated whether altering Wnt/β-catenin signaling might enhance the efficacy of the BRAF(V600E) inhibitor PLX4720. We found that endogenous β-catenin was required for PLX4720-induced apoptosis of melanoma cells and that activation of Wnt/β-catenin signaling synergized with PLX4720 to decrease tumor growth in vivo and to increase apoptosis in vitro. This synergistic enhancement of apoptosis correlated with reduced abundance of an endogenous negative regulator of β-catenin, AXIN1. In support of the hypothesis that AXIN1 is a mediator rather than a marker of apoptosis, siRNA directed against AXIN1 rendered resistant melanoma cell lines susceptible to apoptosis in response to treatment with a BRAF(V600E) inhibitor. Thus, Wnt/β-catenin signaling and AXIN1 may regulate the efficacy of inhibitors of BRAF(V600E), suggesting that manipulation of the Wnt/β-catenin pathway could be combined with BRAF inhibitors to treat melanoma.
Conflict of interest statement
Conflicts of interest: None declared
Figures

Comment in
-
Wnt/β-catenin and MAPK signaling: allies and enemies in different battlefields.Sci Signal. 2012 Apr 10;5(219):pe15. doi: 10.1126/scisignal.2002921. Sci Signal. 2012. PMID: 22494969
References
-
- Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, Davis N, Dicks E, Ewing R, Floyd Y, Gray K, Hall S, Hawes R, Hughes J, Kosmidou V, Menzies A, Mould C, Parker A, Stevens C, Watt S, Hooper S, Wilson R, Jayatilake H, Gusterson BA, Cooper C, Shipley J, Hargrave D, Pritchard-Jones K, Maitland N, Chenevix-Trench G, Riggins GJ, Bigner DD, Palmieri G, Cossu A, Flanagan A, Nicholson A, Ho JW, Leung SY, Yuen ST, Weber BL, Seigler HF, Darrow TL, Paterson H, Marais R, Marshall CJ, Wooster R, Stratton MR, Futreal PA. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949. doi: 10.1038/nature00766. published online EpubJun 27. nature00766 [pii]) - DOI - PubMed
-
- Tsai J, Lee JT, Wang W, Zhang J, Cho H, Mamo S, Bremer R, Gillette S, Kong J, Haass NK, Sproesser K, Li L, Smalley KS, Fong D, Zhu YL, Marimuthu A, Nguyen H, Lam B, Liu J, Cheung I, Rice J, Suzuki Y, Luu C, Settachatgul C, Shellooe R, Cantwell J, Kim SH, Schlessinger J, Zhang KY, West BL, Powell B, Habets G, Zhang C, Ibrahim PN, Hirth P, Artis DR, Herlyn M, Bollag G. Discovery of a selective inhibitor ofoncogenic B-Raf kinase with potent antimelanoma activity. Proc Natl Acad Sci U S A. 2008;105:3041. doi: 10.1073/pnas.0711741105. published online EpubFeb 26. 0711741105 [pii] - DOI - PMC - PubMed
-
- Bollag G, Hirth P, Tsai J, Zhang J, Ibrahim PN, Cho H, Spevak W, Zhang C, Zhang Y, Habets G, Burton EA, Wong B, Tsang G, West BL, Powell B, Shellooe R, Marimuthu A, Nguyen H, Zhang KY, Artis DR, Schlessinger J, Su F, Higgins B, Iyer R, D’Andrea K, Koehler A, Stumm M, Lin PS, Lee RJ, Grippo J, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA, Chapman PB, Flaherty KT, Xu X, Nathanson KL, Nolop K. Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature. 2010;467:596. doi: 10.1038/nature09454. published online EpubSep 30. nature09454 [pii] - DOI - PMC - PubMed
-
- Lee JT, Li L, Brafford PA, van den Eijnden M, Halloran MB, Sproesser K, Haass NK, Smalley KS, Tsai J, Bollag G, Herlyn M. PLX4032, a potent inhibitor of the B-Raf V600Eoncogene, selectively inhibits V600E-positive melanomas. Pigment Cell Melanoma Res. 2010;23:820. doi: 10.1111/j.1755-148X.2010.00763.x. published online EpubDec (PCR763 [pii] - DOI - PMC - PubMed
-
- Kefford RF, Arkenau H, Brown MP, Millward M, Infante JR, Long GV, Ouellet D, Curtis M, Lebowitz PF, Falchook GS. Phase I/II study of GSK2118436, a selective inhibitor of oncogenic mutant BRAF kinase, in patients with metastatic melanoma and other solid tumors. J Clin Oncol. 2010;28:abstract 8503. published online Epub2010 (
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

