Oncogenic viruses and tumor glucose metabolism: like kids in a candy store
- PMID: 22234809
- PMCID: PMC3257817
- DOI: 10.1158/1535-7163.MCT-11-0517
Oncogenic viruses and tumor glucose metabolism: like kids in a candy store
Abstract
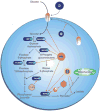
Oncogenic viruses represent a significant public health burden in light of the multitude of malignancies that result from chronic or spontaneous viral infection and transformation. Although many of the molecular signaling pathways that underlie virus-mediated cellular transformation are known, the impact of these viruses on metabolic signaling and phenotype within proliferating tumor cells is less well understood. Whether the interaction of oncogenic viruses with metabolic signaling pathways involves enhanced glucose uptake and glycolysis (both hallmark features of transformed cells) or dysregulation of molecular pathways that regulate oxidative stress, viruses are adept at facilitating tumor expansion. Through their effects on cell proliferation pathways, such as the PI3K and MAPK pathways, the cell cycle regulatory proteins p53 and ATM, and the cell stress response proteins HIF-1α and AMPK, viruses exert control over critical metabolic signaling cascades. Additionally, oncogenic viruses modulate the tumor metabolomic profile through direct and indirect interactions with glucose transporters, such as GLUT1, and specific glycolytic enzymes, including pyruvate kinase, glucose 6-phosphate dehydrogenase, and hexokinase. Through these pathways, oncogenic viruses alter the phenotypic characteristics and energy-use methods of transformed cells; therefore, it may be possible to develop novel antiglycolytic therapies to target these dysregulated pathways in virus-derived malignancies.
©2012 AACR.
Figures

Similar articles
-
A narrative review: exploring viral-induced malignancies through the lens of dysregulated cellular metabolism and glucose transporters.BMC Cancer. 2024 Oct 29;24(1):1329. doi: 10.1186/s12885-024-13013-y. BMC Cancer. 2024. PMID: 39472817 Free PMC article. Review.
-
Metabolic phenotype of bladder cancer.Cancer Treat Rev. 2016 Apr;45:46-57. doi: 10.1016/j.ctrv.2016.03.005. Epub 2016 Mar 8. Cancer Treat Rev. 2016. PMID: 26975021 Review.
-
Orexin A affects HepG2 human hepatocellular carcinoma cells glucose metabolism via HIF-1α-dependent and -independent mechanism.PLoS One. 2017 Sep 8;12(9):e0184213. doi: 10.1371/journal.pone.0184213. eCollection 2017. PLoS One. 2017. PMID: 28886081 Free PMC article.
-
Pathways involved in viral oncogenesis: New perspectives from virus-host protein interactomics.Biochim Biophys Acta Mol Basis Dis. 2020 Oct 1;1866(10):165885. doi: 10.1016/j.bbadis.2020.165885. Epub 2020 Jun 20. Biochim Biophys Acta Mol Basis Dis. 2020. PMID: 32574835
-
Reprogramming of cellular metabolic pathways by human oncogenic viruses.Curr Opin Virol. 2019 Dec;39:60-69. doi: 10.1016/j.coviro.2019.11.002. Epub 2019 Nov 22. Curr Opin Virol. 2019. PMID: 31766001 Free PMC article. Review.
Cited by
-
The novel mechanism facilitating chronic hepatitis B infection: immunometabolism and epigenetic modification reprogramming.Front Immunol. 2024 Jan 15;15:1349867. doi: 10.3389/fimmu.2024.1349867. eCollection 2024. Front Immunol. 2024. PMID: 38288308 Free PMC article. Review.
-
HPV - A different view on Head and Neck Cancer.Laryngorhinootologie. 2018 Mar;97(S 01):S48-S113. doi: 10.1055/s-0043-121596. Epub 2018 Mar 22. Laryngorhinootologie. 2018. PMID: 29905354 Free PMC article. Review.
-
HPV18 oncoproteins driven expression of PKM2 reprograms HeLa cell metabolism to maintain aerobic glycolysis and viability.Virusdisease. 2022 Sep;33(3):223-235. doi: 10.1007/s13337-022-00776-w. Epub 2022 Jul 29. Virusdisease. 2022. PMID: 36277414 Free PMC article.
-
Molecular mechanisms of viral oncogenesis in humans.Nat Rev Microbiol. 2018 Nov;16(11):684-698. doi: 10.1038/s41579-018-0064-6. Nat Rev Microbiol. 2018. PMID: 30143749 Free PMC article. Review.
-
Replication of the Shrimp Virus WSSV Depends on Glutamate-Driven Anaplerosis.PLoS One. 2016 Jan 11;11(1):e0146902. doi: 10.1371/journal.pone.0146902. eCollection 2016. PLoS One. 2016. PMID: 26751681 Free PMC article.
References
-
- Krynska B, Del Valle L, Gordon J, Otte J, Croul S, Khalili K. Identification of a novel p53 mutation in JCV-induced mouse medulloblastoma. Virology. 2000;274:65–74. - PubMed
-
- Giampieri S, Garcia-Escudero R, Green J, Storey A. Human papillomavirus type 77 E6 protein selectively inhibits p53-dependent transcription of proapoptotic genes following UV-B irradiation. Oncogene. 2004;23:5864–70. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

