Structural mechanism of the phosphorylation-dependent dimerization of the MDC1 forkhead-associated domain
- PMID: 22234877
- PMCID: PMC3351156
- DOI: 10.1093/nar/gkr1296
Structural mechanism of the phosphorylation-dependent dimerization of the MDC1 forkhead-associated domain
Abstract
MDC1 is a key mediator of the DNA-damage response in mammals with several phosphorylation-dependent protein interaction domains. The function of its N-terminal forkhead-associated (FHA) domain remains elusive. Here, we show with structural, biochemical and cellular data that the FHA domain mediates phosphorylation-dependent dimerization of MDC1 in response to DNA damage. Crystal structures of the FHA domain reveal a face-to-face dimer with pseudo-dyad symmetry. We found that the FHA domain recognizes phosphothreonine 4 (pT4) at the N-terminus of MDC1 and determined its crystal structure in complex with a pT4 peptide. Biochemical analysis further revealed that in the dimer, the FHA domain binds in trans to pT4 from the other subunit, which greatly stabilizes the otherwise unstable dimer. We show that T4 is phosphorylated primarily by ATM upon DNA damage. MDC1 mutants with the FHA domain deleted or impaired in its ability to dimerize formed fewer foci at DNA-damage sites, but the localization defect was largely rescued by an artificial dimerization module, suggesting that dimerization is the primary function of the MDC1 FHA domain. Our results suggest a novel mechanism for the regulation of MDC1 function through T4 phosphorylation and FHA-mediated dimerization.
Figures

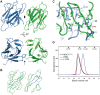

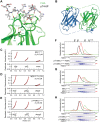
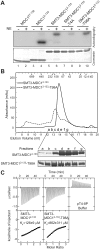
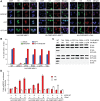
Comment on
- Nucleic Acids Res. doi: 10.1093/nar/gkr1300
References
-
- Ohnishi T, Mori E, Takahashi A. DNA double-strand breaks: their production, recognition, and repair in eukaryotes. Mutat. Res. 2009;669:8–12. - PubMed
-
- Williams RS, Williams JS, Tainer JA. Mre11-Rad50-Nbs1 is a keystone complex connecting DNA repair machinery, double-strand break signaling, and the chromatin template. Biochem. Cell Biol. 2007;85:509–520. - PubMed
-
- Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J. Biol. Chem. 1998;273:5858–5868. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

