Endotoxin priming of neutrophils requires endocytosis and NADPH oxidase-dependent endosomal reactive oxygen species
- PMID: 22235113
- PMCID: PMC3320989
- DOI: 10.1074/jbc.M111.306530
Endotoxin priming of neutrophils requires endocytosis and NADPH oxidase-dependent endosomal reactive oxygen species
Abstract
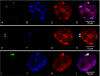
NADPH oxidase 2 (Nox2)-generated reactive oxygen species (ROS) are critical for neutrophil (polymorphonuclear leukocyte (PMN)) microbicidal function. Nox2 also plays a role in intracellular signaling, but the site of oxidase assembly is unknown. It has been proposed to occur on secondary granules. We previously demonstrated that intracellular NADPH oxidase-derived ROS production is required for endotoxin priming. We hypothesized that endotoxin drives Nox2 assembly on endosomes. Endotoxin induced ROS generation within an endosomal compartment as quantified by flow cytometry (dihydrorhodamine 123 and Oxyburst Green). Inhibition of endocytosis by the dynamin-II inhibitor Dynasore blocked endocytosis of dextran, intracellular generation of ROS, and priming of PMN by endotoxin. Confocal microscopy demonstrated a ROS-containing endosomal compartment that co-labeled with gp91(phox), p40(phox), p67(phox), and Rab5, but not with the secondary granule marker CD66b. To further characterize this compartment, PMNs were fractionated by nitrogen cavitation and differential centrifugation, followed by free flow electrophoresis. Specific subfractions made superoxide in the presence of NADPH by cell-free assay (cytochrome c). Subfraction content of membrane and cytosolic subunits of Nox2 correlated with ROS production. Following priming, there was a shift in the light membrane subfractions where ROS production was highest. CD66b was not mobilized from the secondary granule compartment. These data demonstrate a novel, nonphagosomal intracellular site for Nox2 assembly. This compartment is endocytic in origin and is required for PMN priming by endotoxin.
Figures






References
-
- Bass D. A., Olbrantz P., Szejda P., Seeds M. C., McCall C. E. (1986) Subpopulations of neutrophils with increased oxidative product formation in blood of patients with infection. J. Immunol. 136, 860–866 - PubMed
-
- Chollet-Martin S., Montravers P., Gibert C., Elbim C., Desmonts J. M., Fagon J. Y., Gougerot-Pocidalo M. A. (1992) Subpopulation of hyperresponsive polymorphonuclear neutrophils in patients with adult respiratory distress syndrome. Role of cytokine production. Am. Rev. Respir. Dis. 146, 990–996 - PubMed
-
- Ogura H., Tanaka H., Koh T., Hashiguchi N., Kuwagata Y., Hosotsubo H., Shimazu T., Sugimoto H. (1999) Priming, second-hit priming, and apoptosis in leukocytes from trauma patients. J. Trauma 46, 774–783 - PubMed
-
- Condliffe A. M., Kitchen E., Chilvers E. R. (1998) Neutrophil priming. Pathophysiological consequences and underlying mechanisms. Clin. Sci. 94, 461–471 - PubMed
-
- Swain S. D., Rohn T. T., Quinn M. T. (2002) Neutrophil priming in host defense. Role of oxidants as priming agents. Antioxid. Redox Signal. 4, 69–83 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

