Towards a "Sample-In, Answer-Out" Point-of-Care Platform for Nucleic Acid Extraction and Amplification: Using an HPV E6/E7 mRNA Model System
- PMID: 22235204
- PMCID: PMC3253481
- DOI: 10.1155/2012/905024
Towards a "Sample-In, Answer-Out" Point-of-Care Platform for Nucleic Acid Extraction and Amplification: Using an HPV E6/E7 mRNA Model System
Abstract
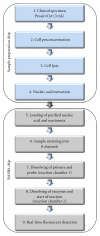
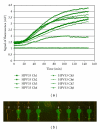
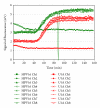
The paper presents the development of a "proof-of-principle" hands-free and self-contained diagnostic platform for detection of human papillomavirus (HPV) E6/E7 mRNA in clinical specimens. The automated platform performs chip-based sample preconcentration, nucleic acid extraction, amplification, and real-time fluorescent detection with minimal user interfacing. It consists of two modular prototypes, one for sample preparation and one for amplification and detection; however, a common interface is available to facilitate later integration into one single module. Nucleic acid extracts (n = 28) from cervical cytology specimens extracted on the sample preparation chip were tested using the PreTect HPV-Proofer and achieved an overall detection rate for HPV across all dilutions of 50%-85.7%. A subset of 6 clinical samples extracted on the sample preparation chip module was chosen for complete validation on the NASBA chip module. For 4 of the samples, a 100% amplification for HPV 16 or 33 was obtained at the 1 : 10 dilution for microfluidic channels that filled correctly. The modules of a "sample-in, answer-out" diagnostic platform have been demonstrated from clinical sample input through sample preparation, amplification and final detection.
Figures



Similar articles
-
PreTect HPV-Proofer: real-time detection and typing of E6/E7 mRNA from carcinogenic human papillomaviruses.J Virol Methods. 2007 Jun;142(1-2):204-12. doi: 10.1016/j.jviromet.2007.01.036. Epub 2007 Mar 26. J Virol Methods. 2007. PMID: 17379322
-
E6/E7 mRNA expression analysis: a test for the objective assessment of cervical adenocarcinoma in clinical prognostic procedure.Int J Oncol. 2010 Jun;36(6):1533-9. doi: 10.3892/ijo_00000640. Int J Oncol. 2010. PMID: 20428778
-
Isolation of RNA from residual BD SurePath liquid-based cytology specimens and detection of HPV E6/E7 mRNA using the PreTectt HPV-Proofer assay.J Virol Methods. 2008 Dec;154(1-2):220-2. doi: 10.1016/j.jviromet.2008.08.002. Epub 2008 Sep 20. J Virol Methods. 2008. PMID: 18761379
-
[Research progress on analysis of human papillomavirus by microchip capillary electrophoresis].Se Pu. 2020 Oct 8;38(10):1179-1188. doi: 10.3724/SP.J.1123.2020.05016. Se Pu. 2020. PMID: 34213114 Review. Chinese.
-
Rapid on-site nucleic acid testing: On-chip sample preparation, amplification, and detection, and their integration into all-in-one systems.Front Bioeng Biotechnol. 2023 Feb 1;11:1020430. doi: 10.3389/fbioe.2023.1020430. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 36815884 Free PMC article. Review.
Cited by
-
Advances in point-of-care nucleic acid extraction technologies for rapid diagnosis of human and plant diseases.Biosens Bioelectron. 2020 Dec 1;169:112592. doi: 10.1016/j.bios.2020.112592. Epub 2020 Sep 8. Biosens Bioelectron. 2020. PMID: 32942143 Free PMC article. Review.
-
Chemical Trends in Sample Preparation for Nucleic Acid Amplification Testing (NAAT): A Review.Biosensors (Basel). 2023 Nov 10;13(11):980. doi: 10.3390/bios13110980. Biosensors (Basel). 2023. PMID: 37998155 Free PMC article. Review.
-
Human papillomavirus molecular biology and disease association.Rev Med Virol. 2015 Mar;25 Suppl 1(Suppl Suppl 1):2-23. doi: 10.1002/rmv.1822. Rev Med Virol. 2015. PMID: 25752814 Free PMC article. Review.
-
Molecular oncology testing in resource-limited settings.J Mol Diagn. 2014 Nov;16(6):601-11. doi: 10.1016/j.jmoldx.2014.07.002. Epub 2014 Sep 19. J Mol Diagn. 2014. PMID: 25242061 Free PMC article. Review.
-
The potential of RNA as a target for national screening of pre-cancer.J Public Health Afr. 2018 Dec 21;9(3):866. doi: 10.4081/jphia.2018.866. eCollection 2018 Dec 21. J Public Health Afr. 2018. PMID: 30854179 Free PMC article.
References
-
- Yager P, Domingo GJ, Gerdes J. Point-of-care diagnostics for global health. Annual Review of Biomedical Engineering. 2008;10:107–144. - PubMed
-
- Mark D, Haeberle S, Roth G, Von Stetten F, Zengerle R. Microfluidic lab-on-a-chip platforms: requirements, characteristics and applications. Chemical Society Reviews. 2010;39(3):1153–1182. - PubMed
-
- Schulte TH, Bardell RL, Weigl BH. Microfluidic technologies in clinical diagnostics. Clinica Chimica Acta. 2002;321(1-2):1–10. - PubMed
-
- Tüdos AJ, Besselink GAJ, Schasfoort RBM. Trends in miniaturized total analysis systems for point-of-care testing in clinical chemistry. Lab on a Chip. 2001;1(2):83–95. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

