Examination of the activity of camel milk casein against hepatitis C virus (genotype-4a) and its apoptotic potential in hepatoma and hela cell lines
- PMID: 22235215
- PMCID: PMC3234544
- DOI: 10.5812/kowsar.1735143X.722
Examination of the activity of camel milk casein against hepatitis C virus (genotype-4a) and its apoptotic potential in hepatoma and hela cell lines
Abstract
Background: Hepatitis C is a global health concern that represents a major cause of liver disease and socioeconomic burden. Currently, there is no vaccine that protects against this infection or drug that treats it effectively. The current treatment for hepatitis C virus (HCV) infection does not produce a sustained virologic response. Therefore,discovery and identification of a new drug for HCV treatment is a high priority.Camel milk is a traditional medicine that could improve the control of HCV.
Objectives: To assess the potential effect of casein purified from camel milk on HCV cellular infectivity in a tissue culture model.
Materials and methods: Casein was purified from defatted camel milk to electrophoretic homogeneity. PBMCs and HepG2 and HeLa cell lines were used. Three kinds of experiments were conducted. HCV was directly interacted with casein and then mixed with different cell types, casein was incubated with the cells and then exposed to HCV, and the HCV pre-infected cells were treated with casein at different concentrations and time intervals. Non-infected cells were used to assess cytotoxicity and the apoptosis effect of casein.
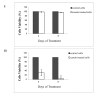
Results: Direct interaction of casein (with or without α-lactalbumin) with neither the virus nor the cells prevented HCV cell entry. However, casein with α-lactalbumin induced a cytotoxic effect in HepG2 and HeLa cell lines but not in human naïve leukocytes. At all concentrations tested, casein with α-lactalbumin could induce apoptosis in both infected and non-infected HepG2 cells.
Conclusions: Camel milk casein (with or without α-lactalbumin) did not demonstrate any anti-HCV activity. However, the cellular apoptotic cascade was initiated in HepG2 and HeLa cells treated with casein (with α-lactalbumin) but not in naïve leukocytes.
Keywords: Apoptosis; Blocking; Camel; Casein; Cell entry; Hepatitis C virus.
Figures
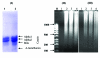


Similar articles
-
Research Development on Anti-Microbial and Antioxidant Properties of Camel Milk and Its Role as an Anti-Cancer and Anti-Hepatitis Agent.Antioxidants (Basel). 2021 May 17;10(5):788. doi: 10.3390/antiox10050788. Antioxidants (Basel). 2021. PMID: 34067516 Free PMC article. Review.
-
Anti-infectivity of camel polyclonal antibodies against hepatitis C virus in Huh7.5 hepatoma.Virol J. 2012 Sep 16;9:201. doi: 10.1186/1743-422X-9-201. Virol J. 2012. PMID: 22978304 Free PMC article.
-
Screening the anti infectivity potentials of native N- and C-lobes derived from the camel lactoferrin against hepatitis C virus.BMC Complement Altern Med. 2014 Jul 3;14:219. doi: 10.1186/1472-6882-14-219. BMC Complement Altern Med. 2014. PMID: 24993815 Free PMC article.
-
Effectiveness of human, camel, bovine and sheep lactoferrin on the hepatitis C virus cellular infectivity: comparison study.Virol J. 2013 Jun 19;10:199. doi: 10.1186/1743-422X-10-199. Virol J. 2013. PMID: 23782993 Free PMC article.
-
Antiviral activities of whey proteins.Appl Microbiol Biotechnol. 2015 Sep;99(17):6997-7008. doi: 10.1007/s00253-015-6818-4. Epub 2015 Jul 22. Appl Microbiol Biotechnol. 2015. PMID: 26198883 Free PMC article. Review.
Cited by
-
Molecular cloning and 3D structure modeling of APEX1, DNA base excision repair enzyme from the Camel, Camelus dromedarius.Int J Mol Sci. 2012;13(7):8578-8596. doi: 10.3390/ijms13078578. Epub 2012 Jul 10. Int J Mol Sci. 2012. PMID: 22942721 Free PMC article.
-
Augmenting apoptosis-mediated anticancer activity of lactoperoxidase and lactoferrin by nanocombination with copper and iron hybrid nanometals.Sci Rep. 2022 Aug 1;12(1):13153. doi: 10.1038/s41598-022-17357-y. Sci Rep. 2022. PMID: 35915221 Free PMC article.
-
Research Development on Anti-Microbial and Antioxidant Properties of Camel Milk and Its Role as an Anti-Cancer and Anti-Hepatitis Agent.Antioxidants (Basel). 2021 May 17;10(5):788. doi: 10.3390/antiox10050788. Antioxidants (Basel). 2021. PMID: 34067516 Free PMC article. Review.
-
Camels' biological fluids contained nanobodies: promising avenue in cancer therapy.Cancer Cell Int. 2022 Sep 7;22(1):279. doi: 10.1186/s12935-022-02696-7. Cancer Cell Int. 2022. PMID: 36071488 Free PMC article. Review.
-
Therapeutic Effect of Camel Milk and Its Exosomes on MCF7 Cells In Vitro and In Vivo.Integr Cancer Ther. 2018 Dec;17(4):1235-1246. doi: 10.1177/1534735418786000. Epub 2018 Jul 10. Integr Cancer Ther. 2018. PMID: 29986606 Free PMC article.
References
-
- Hoofnagle JH. Management of hepatitis C: current and future perspectives. J Hepatol. 1999;31(Suppl 1):264–8. - PubMed
-
- el-Awady MK, Tabll AA, el-Abd YS, Bahgat MM, Shoeb HA, Youssef SS, Bader el-Din NG, Redwan el-RM, el-Demellawy M, Omran MH, el-Garf WT, Goueli SA. HepG2 cells support viral replication and gene expression of hepatitis C virus genotype 4 in vitro. World J Gastroenterol. 2006;12(30):4836–42. - PMC - PubMed
-
- Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358(9286):958–65. - PubMed
-
- Redwan e-RM, Tabll A. Camel Lactoferrin Markedly Inhibits Hepatitis C Virus Genotype 4 Infection of Human Peripheral Blood Leukocytes. J Immunoassay Immunochem. 2007;28(3):267–77. - PubMed
LinkOut - more resources
Full Text Sources
