IgG placental transfer in healthy and pathological pregnancies
- PMID: 22235228
- PMCID: PMC3251916
- DOI: 10.1155/2012/985646
IgG placental transfer in healthy and pathological pregnancies
Abstract
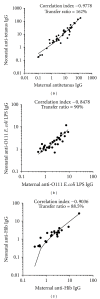
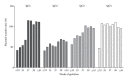
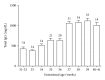
Placental transfer of maternal IgG antibodies to the fetus is an important mechanism that provides protection to the infant while his/her humoral response is inefficient. IgG is the only antibody class that significantly crosses the human placenta. This crossing is mediated by FcRn expressed on syncytiotrophoblast cells. There is evidence that IgG transfer depends on the following: (i) maternal levels of total IgG and specific antibodies, (ii) gestational age, (iii) placental integrity, (iv) IgG subclass, and (v) nature of antigen, being more intense for thymus-dependent ones. These features represent the basis for maternal immunization strategies aimed at protecting newborns against neonatal and infantile infectious diseases. In some situations, such as mothers with primary immunodeficiencies, exogenous IgG acquired by intravenous immunoglobulin therapy crosses the placenta in similar patterns to endogenous immunoglobulins and may also protect the offspring from infections in early life. Inversely, harmful autoantibodies may cross the placenta and cause transitory autoimmune disease in the neonate.
Figures

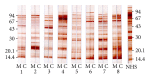
References
-
- Maródi L. Innate cellular immune responses in newborns. Clinical Immunology. 2006;118(2-3):137–144. - PubMed
-
- Levy O. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nature Reviews Immunology. 2007;7(5):379–390. - PubMed
-
- Koyama M, Ito S, Nakajima A, et al. Elevations of group II phospholipase A2 concentrations in serum and amniotic fluid in association with preterm labor. American Journal of Obstetrics and Gynecology. 2000;183(6):1537–1543. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

