Denervation causes fiber atrophy and myosin heavy chain co-expression in senescent skeletal muscle
- PMID: 22235261
- PMCID: PMC3250397
- DOI: 10.1371/journal.pone.0029082
Denervation causes fiber atrophy and myosin heavy chain co-expression in senescent skeletal muscle
Abstract
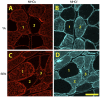
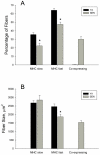
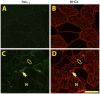
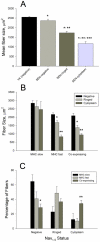
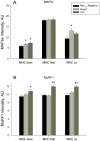
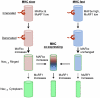
Although denervation has long been implicated in aging muscle, the degree to which it is causes the fiber atrophy seen in aging muscle is unknown. To address this question, we quantified motoneuron soma counts in the lumbar spinal cord using choline acetyl transferase immunhistochemistry and quantified the size of denervated versus innervated muscle fibers in the gastrocnemius muscle using the in situ expression of the denervation-specific sodium channel, Nav₁.₅, in young adult (YA) and senescent (SEN) rats. To gain insights into the mechanisms driving myofiber atrophy, we also examined the myofiber expression of the two primary ubiquitin ligases necessary for muscle atrophy (MAFbx, MuRF1). MN soma number in lumbar spinal cord declined 27% between YA (638±34 MNs×mm⁻¹) and SEN (469±13 MNs×mm⁻¹). Nav₁.₅ positive fibers (1548±70 μm²) were 35% smaller than Nav₁.₅ negative fibers (2367±78 μm²; P<0.05) in SEN muscle, whereas Nav₁.₅ negative fibers in SEN were only 7% smaller than fibers in YA (2553±33 μm²; P<0.05) where no Nav₁.₅ labeling was seen, suggesting denervation is the primary cause of aging myofiber atrophy. Nav₁.₅ positive fibers had higher levels of MAFbx and MuRF1 (P<0.05), consistent with involvement of the proteasome proteolytic pathway in the atrophy of denervated muscle fibers in aging muscle. In summary, our study provides the first quantitative assessment of the contribution of denervation to myofiber atrophy in aging muscle, suggesting it explains the majority of the atrophy we observed. This striking result suggests a renewed focus should be placed on denervation in seeking understanding of the causes of and treatments for aging muscle atrophy.
Conflict of interest statement
Figures


References
-
- Hepple RT. Sci Aging Knowledge Environ 2003: pe31; 2003. Sarcopenia-A critical perspective.pe31 - PubMed
-
- Faulkner JA, Larkin LM, Claflin DR, Brooks SV. Age-related changes in the structure and function of skeletal muscles. Clinical and experimental pharmacology & physiology. 2007;34:1091–1096. - PubMed
-
- Edstrom E, Altun M, Bergman E, Johnson H, Kullberg S, et al. Factors contributing to neuromuscular impairment and sarcopenia during aging. Physiology & Behavior. 2007;92:129–135. - PubMed
-
- Gutmann E, Hanzlikova V. Motor unit in old age. Nature. 1966;209:921–922. - PubMed
-
- Tomlinson BE, Irving D. The numbers of limb motor neurons in the human lumbosacral cord throughout life. JNeurolSci. 1977;34:213–219. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

