Challenge of pigs with classical swine fever viruses after C-strain vaccination reveals remarkably rapid protection and insights into early immunity
- PMID: 22235283
- PMCID: PMC3250419
- DOI: 10.1371/journal.pone.0029310
Challenge of pigs with classical swine fever viruses after C-strain vaccination reveals remarkably rapid protection and insights into early immunity
Abstract
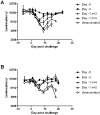
Pre-emptive culling is becoming increasingly questioned as a means of controlling animal diseases, including classical swine fever (CSF). This has prompted discussions on the use of emergency vaccination to control future CSF outbreaks in domestic pigs. Despite a long history of safe use in endemic areas, there is a paucity of data on aspects important to emergency strategies, such as how rapidly CSFV vaccines would protect against transmission, and if this protection is equivalent for all viral genotypes, including highly divergent genotype 3 strains. To evaluate these questions, pigs were vaccinated with the Riemser® C-strain vaccine at 1, 3 and 5 days prior to challenge with genotype 2.1 and 3.3 challenge strains. The vaccine provided equivalent protection against clinical disease caused by for the two challenge strains and, as expected, protection was complete at 5 days post-vaccination. Substantial protection was achieved after 3 days, which was sufficient to prevent transmission of the 3.3 strain to animals in direct contact. Even by one day post-vaccination approximately half the animals were partially protected, and were able to control the infection, indicating that a reduction of the infectious potential is achieved very rapidly after vaccination. There was a close temporal correlation between T cell IFN-γ responses and protection. Interestingly, compared to responses of animals challenged 5 days after vaccination, challenge of animals 3 or 1 days post-vaccination resulted in impaired vaccine-induced T cell responses. This, together with the failure to detect a T cell IFN-γ response in unprotected and unvaccinated animals, indicates that virulent CSFV can inhibit the potent antiviral host defences primed by C-strain in the early period post vaccination.
Conflict of interest statement
Figures

References
-
- van Oirschot JT. Vaccinology of classical swine fever: from lab to field. Vet Microbiol. 2003;96:367–384. - PubMed
-
- Suradhat S, Damrongwatanapokin S, Thanawongnuwech R. Factors critical for successful vaccination against classical swine fever in endemic areas. Vet Microbiol. 2007;119:1–9. - PubMed
-
- van Oirschot JT. Emergency vaccination against classical swine fever. Dev Biol (Basel) 2003;114:259–267. - PubMed
-
- Vandeputte J, Chappuis G. Classical swine fever: the European experience and a guide for infected areas. Rev Sci Tech. 1999;18:638–647. - PubMed
-
- von Ruden S, Staubach C, Kaden V, Hess RG, Blicke J, et al. Retrospective analysis of the oral immunisation of wild boar populations against classical swine fever virus (CSFV) in region Eifel of Rhineland-Palatinate. Vet Microbiol. 2008;132:29–38. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

