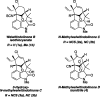
A unified route to the welwitindolinone alkaloids: total syntheses of (-)-N-methylwelwitindolinone C isothiocyanate, (-)-N-methylwelwitindolinone C isonitrile, and (-)-3-hydroxy-N-methylwelwitindolinone C isothiocyanate
- PMID: 22235963
- PMCID: PMC3368435
- DOI: 10.1021/ja210793x
A unified route to the welwitindolinone alkaloids: total syntheses of (-)-N-methylwelwitindolinone C isothiocyanate, (-)-N-methylwelwitindolinone C isonitrile, and (-)-3-hydroxy-N-methylwelwitindolinone C isothiocyanate
Abstract
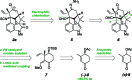
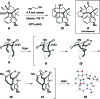
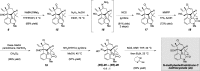
As part of a comprehensive strategy to the welwitindolinone alkaloids possessing a bicyclo[4.3.1]decane core, we report herein concise asymmetric total syntheses of (-)-N-methylwelwitindolinone C isothiocyanate (2a), (-)-N-methylwelwitindolinone C isonitrile (2b), and (-)-3-hydroxy-N-methylwelwitindolinone C isothiocyanate (3a) from a common tetracyclic intermediate. The crucial vinyl chloride moiety was installed through electrophilic chlorination of a hydrazone, but only after adjustment of reactivity to circumvent a facile skeletal rearrangement. Selective desulfurization and oxidation of 2a provided access to 2b and 3a, respectively. Notably, this work provides corrected (1)H and (13)C NMR spectral data for 3a.
© 2011 American Chemical Society
Figures
Similar articles
-
Formal syntheses of naturally occurring welwitindolinones.Org Lett. 2012 Aug 3;14(15):3834-7. doi: 10.1021/ol301424h. Epub 2012 Jul 25. Org Lett. 2012. PMID: 22830424 Free PMC article.
-
Enantiospecific total synthesis of N-methylwelwitindolinone D isonitrile.Angew Chem Int Ed Engl. 2013 Nov 18;52(47):12422-5. doi: 10.1002/anie.201307464. Epub 2013 Oct 2. Angew Chem Int Ed Engl. 2013. PMID: 24123612 Free PMC article.
-
Total synthesis of N-methylwelwitindolinone D isonitrile.J Am Chem Soc. 2011 Apr 20;133(15):5798-801. doi: 10.1021/ja201834u. Epub 2011 Mar 29. J Am Chem Soc. 2011. PMID: 21446729 Free PMC article.
-
[Stereocontrolled Total Synthesis of Biologically Active Natural Products].Yakugaku Zasshi. 2018;138(2):191-209. doi: 10.1248/yakushi.17-00187. Yakugaku Zasshi. 2018. PMID: 29386433 Review. Japanese.
-
Structural and stereochemical diversity in prenylated indole alkaloids containing the bicyclo[2.2.2]diazaoctane ring system from marine and terrestrial fungi.Nat Prod Rep. 2018 Jun 20;35(6):532-558. doi: 10.1039/c7np00042a. Nat Prod Rep. 2018. PMID: 29632911 Free PMC article. Review.
Cited by
-
Formal syntheses of naturally occurring welwitindolinones.Org Lett. 2012 Aug 3;14(15):3834-7. doi: 10.1021/ol301424h. Epub 2012 Jul 25. Org Lett. 2012. PMID: 22830424 Free PMC article.
-
Naturally Occurring Organohalogen Compounds-A Comprehensive Review.Prog Chem Org Nat Prod. 2023;121:1-546. doi: 10.1007/978-3-031-26629-4_1. Prog Chem Org Nat Prod. 2023. PMID: 37488466 Review.
-
Structural diversification of hapalindole and fischerindole natural products via cascade biocatalysis.ACS Catal. 2021 Apr 16;11(8):4670-4681. doi: 10.1021/acscatal.0c05656. Epub 2021 Apr 5. ACS Catal. 2021. PMID: 34354850 Free PMC article.
-
Enantiospecific total synthesis of N-methylwelwitindolinone D isonitrile.Angew Chem Int Ed Engl. 2013 Nov 18;52(47):12422-5. doi: 10.1002/anie.201307464. Epub 2013 Oct 2. Angew Chem Int Ed Engl. 2013. PMID: 24123612 Free PMC article.
-
Recent advances in hapalindole-type cyanobacterial alkaloids: biosynthesis, synthesis, and biological activity.Nat Prod Rep. 2021 Sep 23;38(9):1567-1588. doi: 10.1039/d1np00007a. Nat Prod Rep. 2021. PMID: 34032254 Free PMC article. Review.
References
-
- Stratmann K.; Moore R. E.; Bonjouklian R.; Deeter J. B.; Patterson G. M. L.; Shaffer S.; Smith C. D.; Smitka T. A. J. Am. Chem. Soc. 1994, 116, 9935.
- Jimenez J. I.; Huber U.; Moore R. E.; Patterson G. M. L. J. Nat. Prod. 1999, 62, 569. - PubMed
-
- Konopelski J. P.; Deng H.; Schiemann K.; Keane J. M.; Olmstead M. M. Synlett 1998, 1105.
- Wood J. L.; Holubec A. A.; Stoltz B. M.; Weiss M. M.; Dixon J. A.; Doan B. D.; Shamji M. F.; Chen J. M.; Heffron T. P. J. Am. Chem. Soc. 1999, 121, 6326.
- Deng H.; Konopelski J. P. Org. Lett. 2001, 3, 3001. - PubMed
- Jung M. E.; Slowinski F. Tetrahedron Lett. 2001, 42, 6835.
- López-Alvarado P.; García-Granda S.; Àlvarez-Rúa C.; Avendaño C. Eur. J. Org. Chem. 2002, 1702.
- Ready J. M.; Reisman S. E.; Hirata M.; Weiss M. M.; Tamaki K.; Ovaska T. V.; Wood J. L. Angew. Chem., Int. Ed. 2004, 43, 1270. - PubMed
- MacKay J. A.; Bishop R. L.; Rawal V. H. Org. Lett. 2005, 7, 3421. - PubMed
- Baudoux J.; Blake A. J.; Simpkins N. S. Org. Lett. 2005, 7, 4087. - PubMed
- Greshock T. J.; Funk R. L. Org. Lett. 2006, 8, 2643. - PMC - PubMed
- Lauchli R.; Shea K. J. Org. Lett. 2006, 8, 5287. - PubMed
- Xia J.; Brown L. E.; Konopelski J. P. J. Org. Chem. 2007, 72, 6885. - PMC - PubMed
- Richter J. M.; Ishihara Y.; Masuda T.; Whitefield B. W.; Llamas T.; Pohjakallio A.; Baran P. S. J. Am. Chem. Soc. 2008, 130, 17938. - PMC - PubMed
- Boissel V.; Simpkins N. S.; Bhalay G.; Blake A. J.; Lewis W. Chem. Commun. 2009, 1398. - PubMed
- Boissel V.; Simpkins N. S.; Bhalay G. Tetrahedron Lett. 2009, 50, 3283.
- Tian X.; Huters A. D.; Douglas C. J.; Garg N. K. Org. Lett. 2009, 11, 2349. - PubMed
- Trost B. M.; McDougall P. J. Org. Lett. 2009, 11, 3782. - PMC - PubMed
- Brailsford J. A.; Lauchli R.; Shea K. J. Org. Lett. 2009, 11, 5330. - PMC - PubMed
- Freeman D. B.; et al. Tetrahedron 2010, 66, 6647. - PMC - PubMed
- Heidebrecht R. W. Jr.; Gulledge B.; Martin S. F. Org. Lett. 2010, 12, 2492. - PMC - PubMed
- Ruiz M.; López-Alvarado P.; Menéndez J. C. Org. Biomol. Chem. 2010, 8, 4521. - PubMed
- Bhat V.; MacKay J. A.; Rawal V. H. Org. Lett. 2011, 13, 3214. - PMC - PubMed
- Bhat V.; MacKay J. A.; Rawal V. H. Tetrahedron 2011, 67, 10097. - PMC - PubMed
-
-
Two elegant syntheses of the unique, cyclobutane-containing member of the family, welwitindolinone A isonitrile, have been reported:
- Baran P. S.; Richter J. M. J. Am. Chem. Soc. 2005, 127, 15394. - PubMed
- Reisman S. E.; Ready J. M.; Hasuoka A.; Smith C. J.; Wood J. L. J. Am. Chem. Soc. 2006, 128, 1448. - PubMed
-
-
- Bhat V.; Rawal V. H. Chem. Commun. 2011, 47, 9705. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials