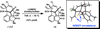Total synthesis of oxidized welwitindolinones and (-)-N-methylwelwitindolinone C isonitrile
- PMID: 22235964
- PMCID: PMC3266994
- DOI: 10.1021/ja210837b
Total synthesis of oxidized welwitindolinones and (-)-N-methylwelwitindolinone C isonitrile
Abstract
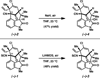
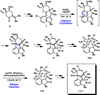
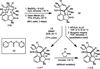
We report the total synthesis of (-)-N-methylwelwitindolinone C isonitrile, in addition to the total syntheses of the 3-hydroxylated welwitindolinones. Our routes to these elusive natural products feature the strategic use of a deuterium kinetic isotope effect to improve the efficiency of a late-stage nitrene insertion reaction. We also provide a computational prediction for the stereochemical configuration at C3 of the hydroxylated welwitindolinones, which was confirmed by experimental studies.
© 2011 American Chemical Society
Figures
Similar articles
-
Formal syntheses of naturally occurring welwitindolinones.Org Lett. 2012 Aug 3;14(15):3834-7. doi: 10.1021/ol301424h. Epub 2012 Jul 25. Org Lett. 2012. PMID: 22830424 Free PMC article.
-
Enantiospecific total synthesis of N-methylwelwitindolinone D isonitrile.Angew Chem Int Ed Engl. 2013 Nov 18;52(47):12422-5. doi: 10.1002/anie.201307464. Epub 2013 Oct 2. Angew Chem Int Ed Engl. 2013. PMID: 24123612 Free PMC article.
-
A unified route to the welwitindolinone alkaloids: total syntheses of (-)-N-methylwelwitindolinone C isothiocyanate, (-)-N-methylwelwitindolinone C isonitrile, and (-)-3-hydroxy-N-methylwelwitindolinone C isothiocyanate.J Am Chem Soc. 2012 Jan 25;134(3):1392-5. doi: 10.1021/ja210793x. Epub 2012 Jan 11. J Am Chem Soc. 2012. PMID: 22235963 Free PMC article.
-
Strategies towards the synthesis of calyciphylline A-type Daphniphyllum alkaloids.Nat Prod Rep. 2014 Apr;31(4):550-62. doi: 10.1039/c3np70115h. Epub 2014 Mar 5. Nat Prod Rep. 2014. PMID: 24595901 Review.
-
Recent Advances on the Total Syntheses of Communesin Alkaloids and Perophoramidine.Chemistry. 2015 Nov 9;21(46):16318-43. doi: 10.1002/chem.201501735. Epub 2015 Sep 10. Chemistry. 2015. PMID: 26353936 Free PMC article. Review.
Cited by
-
Computational predictions of substituted benzyne and indolyne regioselectivities.Tetrahedron Lett. 2015 Jun 3;56(23):3511-3514. doi: 10.1016/j.tetlet.2015.01.022. Tetrahedron Lett. 2015. PMID: 26034336 Free PMC article.
-
Identification and characterization of a welwitindolinone alkaloid biosynthetic gene cluster in the stigonematalean Cyanobacterium Hapalosiphon welwitschii.Chembiochem. 2014 Mar 21;15(5):665-9. doi: 10.1002/cbic.201300794. Chembiochem. 2014. PMID: 24677572 Free PMC article.
-
Photomediated reductive coupling of nitroarenes with aldehydes for amide synthesis.Chem Sci. 2022 Aug 3;13(32):9361-9365. doi: 10.1039/d2sc03047k. eCollection 2022 Aug 17. Chem Sci. 2022. PMID: 36093005 Free PMC article.
-
Total synthesis: Welwitindolinone is well worth it.Nat Chem. 2012 Apr 23;4(5):341-3. doi: 10.1038/nchem.1335. Nat Chem. 2012. PMID: 22522248 No abstract available.
-
Glycal Metallanitrenes for 2-Amino Sugar Synthesis: Amidoglycosylation of Gulal-, Allal-, Glucal-, and Galactal 3-Carbamates.J Org Chem. 2018 Aug 3;83(15):8054-8080. doi: 10.1021/acs.joc.8b00893. Epub 2018 Jul 6. J Org Chem. 2018. PMID: 29979042 Free PMC article.
References
-
- Stratmann K, Moore RE, Bonjouklian R, Deeter JB, Patterson GML, Shaffer S, Smith CD, Smitka TA. J. Am. Chem. Soc. 1994;116:9935–9942.
- Jimenez JL, Huber U, Moore RE, Patterson GML. J. Nat. Prod. 1999;62:569–572. - PubMed
-
-
Welwitindolinone A isonitrile a unique welwitindolinone that possesses a C3 spirooxindoline core has been synthesized independently by the Baran and Wood groups see: Baran PS, Richter JM. J. Am. Chem. Soc. 2005;127:15394–15396. Reisman SE, Ready JM, Hasuoka A, Smith CJ, Wood JL. J. Am. Chem. Soc. 2006;128:1448–1449.
-
-
- Konopelski JP, Deng H, Schiemann K, Keane JM, Olmstead MM. Synlett. 1998:1105–1107.
- Wood JL, Holubec AA, Stoltz BM, Weiss MM, Dixon JA, Doan BD, Shamji MF, Chen JM, Heffron TP. J. Am. Chem. Soc. 1999;121:6326–6327.
- Kaoudi T, Ouiclet-Sire B, Seguin S, Zard SZ. Angew. Chem. Int. Ed. 2000;39:731–733. - PubMed
- Deng H, Konopelski JP. Org. Lett. 2001;3:3001–3004. - PubMed
- Jung ME, Slowinski F. Tetrahedron Lett. 2001;42:6835–6838.
- López-Alvarado P, García-Granda S, Ivarez-Rúa C, Avendaño C. Eur. J. Org. Chem. 2002:1702–1707.
- MacKay JA, Bishop RL, Rawal VH. Org. Lett. 2005;7:3421–3424. - PubMed
- Baudoux J, Blake AJ, Simpkins NS. Org. Lett. 2005;7:4087–4089. - PubMed
- Greshock TJ, Funk RL. Org. Lett. 2006;8:2643–2645. - PMC - PubMed
- Lauchli R, Shea KJ. Org. Lett. 2006;8:5287–5289. - PubMed
- Guthikonda K, Caliando BJ, Du Bois J. Abstracts of Papers. 232nd ACS National Meeting, September 2006, abstr ORGN-002.
- Xia J, Brown LE, Konopelski JP. J. Org. Chem. 2007;72:6885–6890. - PMC - PubMed
- Richter JM, Ishihara Y, Masuda T, Whitefield BW, Llamas T, Pohjakallio A, Baran PS. J. Am. Chem. Soc. 2008;130:17938–17945. - PMC - PubMed
- Boissel V, Simpkins NS, Bhalay G, Blake AJ, Lewis W. Chem. Commun. 2009:1398–1400. - PubMed
- Boissel V, Simpkins NS, Bhalay G. Tetrahedron Lett. 2009;50:3283–3286.
- Tian X, Huters AD, Douglas CJ, Garg NK. Org. Lett. 2009;11:2349–2351. - PubMed
- Trost BM, McDougall PJ. Org. Lett. 2009;11:3782–3785. - PMC - PubMed
- Brailsford JA, Lauchli R, Shea KJ. Org. Lett. 2009;11:5330–5333. - PMC - PubMed
- Freeman DB, et al. Tetrahedron. 2010;66:6647–6655. - PMC - PubMed
- Heidebrecht RW, Jr, Gulledge B, Martin SF. Org. Lett. 2010;12:2492–2495. - PMC - PubMed
- Ruiz M, López-Alvarado P, Menéndez JC. Org. Biomol. Chem. 2010;8:4521–4523. - PubMed
- Bhat V, Rawal VH. Chem. Comm. 2011;47:9705–9707. - PubMed
- Bhat V, MacKay JA, Rawal VH. Org. Lett. 2011;13:3214–3217. - PMC - PubMed
- Bhat V, MacKay JA, Rawal VH. Tetrahedron. 2011;67:10097–10104. - PMC - PubMed
-
-
For pertinent reviews see: Brown LE, Konopelski JP. Org. Prep. Proc. Intl. 2008;40:411–445. Avendaño C, Menéndez JC. Curr. Org. Synth. 2004;1:65–82.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous