Plasticity of mouse enteric synapses mediated through endocannabinoid and purinergic signaling
- PMID: 22235973
- PMCID: PMC3276688
- DOI: 10.1111/j.1365-2982.2011.01860.x
Plasticity of mouse enteric synapses mediated through endocannabinoid and purinergic signaling
Abstract
Background: The enteric nervous system (ENS) possesses extensive synaptic connections which integrate information and provide appropriate outputs to coordinate the activity of the gastrointestinal tract. The regulation of enteric synapses is not well understood. Cannabinoid (CB)(1) receptors inhibit the release of acetylcholine (ACh) in the ENS, but their role in the synapse is not understood. We tested the hypothesis that enteric CB(1) receptors provide inhibitory control of excitatory neurotransmission in the ENS.
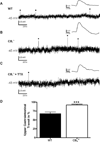
Methods: Intracellular microelectrode recordings were obtained from mouse myenteric plexus neurons. Interganglionic fibers were stimulated with a concentric stimulating electrode to elicit synaptic events on to the recorded neuron. Differences between spontaneous and evoked fast synaptic transmission was examined within preparations from CB(1) deficient mice (CB(1)(-/-)) and wild-type (WT) littermate controls.
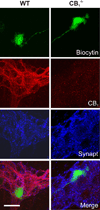
Key results: Cannabinoid receptors were colocalized on terminals expressing the vesicular ACh transporter and the synaptic protein synaptotagmin. A greater proportion of CB(1)(-/-) neurons received spontaneous fast excitatory postsynaptic potentials than neurons from WT preparations. The CB(1) agonist WIN55,212 depressed WT synapses without any effect on CB(1)(-/-) synapses. Synaptic activity in response to depolarization was markedly enhanced at CB(1)(-/-) synapses and after treatment with a CB(1) antagonist in WT preparations. Activity-dependent liberation of a retrograde purine messenger was demonstrated to facilitate synaptic transmission in CB(1)(-/-) mice.
Conclusions & inferences: Cannabinoid receptors inhibit transmitter release at enteric synapses and depress synaptic strength basally and in an activity-dependent manner. These actions help explain accelerated intestinal transit observed in the absence of CB(1) receptors.
© 2012 Blackwell Publishing Ltd.
Figures




References
-
- Furness JB. The enteric nervous system. Malden, Mass: Blackwell Pub; 2006.
-
- Galligan JJ, North RA. Pharmacology and function of nicotinic acetylcholine and P2X receptors in the enteric nervous system. Neurogastroenterol Motil. 2004;16 Suppl 1:64–70. - PubMed
-
- Lomax AE, Sharkey KA, Furness JB. The participation of the sympathetic innervation of the gastrointestinal tract in disease states. Neurogastroenterol Motil. 2010;22:7–18. - PubMed
-
- Wilson RI, Nicoll RA. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature. 2001;410:588–592. - PubMed
-
- Kreitzer AC, Regehr WG. Retrograde inhibition of presynaptic calcium influx by endogenous cannabinoids at excitatory synapses onto Purkinje cells. Neuron. 2001;29:717–727. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

