Hypochlorous acid regulates neutrophil extracellular trap release in humans
- PMID: 22236002
- PMCID: PMC3278692
- DOI: 10.1111/j.1365-2249.2011.04518.x
Hypochlorous acid regulates neutrophil extracellular trap release in humans
Abstract
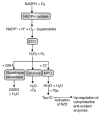
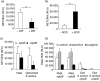
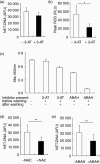
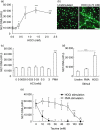
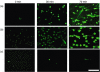
Neutrophil extracellular traps (NETs) comprise extracellular chromatin and granule protein complexes that immobilize and kill bacteria. NET release represents a recently discovered, novel anti-microbial strategy regulated non-exclusively by nicotinamide adenine dinucleotide phosphate (NADPH) oxidase generation of reactive oxygen intermediates (ROIs), particularly hydrogen peroxide. This study aimed to characterize the role of ROIs in the process of NET release and to identify the dominant ROI trigger. We employed various enzymes, inhibitors and ROIs to record their effect fluorometrically on in vitro NET release by human peripheral blood neutrophils. Treatment with exogenous superoxide dismutase (SOD) supported the established link between hydrogen peroxide and NET production. However, treatment with myeloperoxidase inhibitors and direct addition of hypochlorous acid (HOCl; generated in situ from sodium hypochlorite) established that HOCl was a necessary and sufficient ROI for NET release. This was confirmed by the ability of HOCl to stimulate NET release in chronic granulomatous disease (CGD) patient neutrophils which, due to the lack of a functional NADPH oxidase, also lack the capacity for NET release in response to classical stimuli. Moreover, the exogenous addition of taurine, abundantly present within the neutrophil cytosol, abrogated NET production stimulated by phorbol myristate acetate (PMA) and HOCl, providing a novel mode of cytoprotection by taurine against oxidative stress by taurine.
© 2012 The Authors. Clinical and Experimental Immunology © 2012 British Society for Immunology.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

