Comparison of interferon-γ-, interleukin (IL)-17- and IL-22-expressing CD4 T cells, IL-22-expressing granulocytes and proinflammatory cytokines during latent and active tuberculosis infection
- PMID: 22236009
- PMCID: PMC3278699
- DOI: 10.1111/j.1365-2249.2011.04520.x
Comparison of interferon-γ-, interleukin (IL)-17- and IL-22-expressing CD4 T cells, IL-22-expressing granulocytes and proinflammatory cytokines during latent and active tuberculosis infection
Abstract
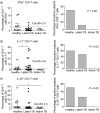
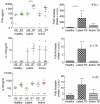
In this study, we investigated the role and expression of T helper type 17 (Th17) cells and Th17 cytokines in human tuberculosis. We show that the basal proportion of interferon (IFN)-γ-, interleukin (IL)-17- and IL-22-expressing CD4(+) T cells and IL-22-expressing granulocytes in peripheral blood were significantly lower in latently infected healthy individuals and active tuberculosis patients compared to healthy controls. In contrast, CD4(+) T cells expressing IL-17, IL-22 and IFN-γ were increased significantly following mycobacterial antigens stimulation in both latent and actively infected patients. Interestingly, proinflammatory IFN-γ and tumour necrosis factor (TNF)-α were increased following antigen stimulation in latent infection. Similarly, IL-1β, IL-4, IL-8, IL-22 and TNF-α were increased in the serum of latently infected individuals, whereas IL-6 and TNF-α were increased significantly in actively infected patients. Overall, we observed differential induction of IL-17-, IL-22- and IFN-γ-expressing CD4(+) T cells, IL-22-expressing granulocytes and proinflammatory cytokines in circulation and following antigenic stimulation in latent and active tuberculosis.
© 2012 The Authors. Clinical and Experimental Immunology © 2012 British Society for Immunology.
Figures




Similar articles
-
Circulating mycobacterial-reactive CD4+ T cells with an immunosuppressive phenotype are higher in active tuberculosis than latent tuberculosis infection.Tuberculosis (Edinb). 2014 Sep;94(5):494-501. doi: 10.1016/j.tube.2014.07.002. Epub 2014 Jul 17. Tuberculosis (Edinb). 2014. PMID: 25095750
-
Multi-functional flow cytometry analysis of CD4+ T cells as an immune biomarker for latent tuberculosis status in patients treated with tumour necrosis factor (TNF) antagonists.Clin Exp Immunol. 2014 Jun;176(3):410-7. doi: 10.1111/cei.12290. Clin Exp Immunol. 2014. PMID: 24528189 Free PMC article.
-
Study of CD27 and CCR4 Markers on Specific CD4+ T-Cells as Immune Tools for Active and Latent Tuberculosis Management.Front Immunol. 2019 Jan 9;9:3094. doi: 10.3389/fimmu.2018.03094. eCollection 2018. Front Immunol. 2019. PMID: 30687314 Free PMC article.
-
Functional profile of CD4+ and CD8+ T cells in latently infected individuals and patients with active TB.Tuberculosis (Edinb). 2013 Mar;93(2):155-66. doi: 10.1016/j.tube.2012.12.002. Epub 2013 Jan 16. Tuberculosis (Edinb). 2013. PMID: 23332142
-
Rv2204c, Rv0753c and Rv0009 antigens specific T cell responses in latent and active TB - a flow cytometry-based analysis.Int J Med Microbiol. 2018 Mar;308(2):297-305. doi: 10.1016/j.ijmm.2017.12.001. Epub 2017 Dec 6. Int J Med Microbiol. 2018. PMID: 29325881
Cited by
-
Latent tuberculosis infection is associated with increased unstimulated levels of interferon-gamma in Lima, Peru.PLoS One. 2018 Sep 13;13(9):e0202191. doi: 10.1371/journal.pone.0202191. eCollection 2018. PLoS One. 2018. PMID: 30212453 Free PMC article. Clinical Trial.
-
Peripheral artery disease: epidemiology and global perspectives.Nat Rev Cardiol. 2017 Mar;14(3):156-170. doi: 10.1038/nrcardio.2016.179. Epub 2016 Nov 17. Nat Rev Cardiol. 2017. PMID: 27853158 Review.
-
Characterization of immune responses of human PBMCs infected with Mycobacterium tuberculosis H37Ra: Impact of donor declared BCG vaccination history on immune responses and M. tuberculosis growth.PLoS One. 2018 Sep 11;13(9):e0203822. doi: 10.1371/journal.pone.0203822. eCollection 2018. PLoS One. 2018. PMID: 30204787 Free PMC article.
-
Innate lymphoid cells exhibited IL-17-expressing phenotype in active tuberculosis disease.BMC Pulm Med. 2021 Oct 12;21(1):318. doi: 10.1186/s12890-021-01678-1. BMC Pulm Med. 2021. PMID: 34641843 Free PMC article.
-
Role of latent tuberculosis infection on elevated risk of cardiovascular disease: a population-based cohort study of immigrants in British Columbia, Canada, 1985-2019.Epidemiol Infect. 2023 Apr 17;151:e68. doi: 10.1017/S0950268823000559. Epidemiol Infect. 2023. PMID: 37066967 Free PMC article.
References
-
- Dye C, Scheele S, Dolin P, Pathania V, Raviglione MC. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA. 1999;282:677–86. - PubMed
-
- Cooper AM, Kipnis A, Turner J, Magram J, Ferrante J, Orme IM. Mice lacking bioactive IL-12 can generate protective, antigen-specific cellular responses to mycobacterial infection only if the IL-12 p40 subunit is present. J Immunol. 2002;168:1322–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

