Differential regulation of acid sphingomyelinase in macrophages stimulated with oxidized low-density lipoprotein (LDL) and oxidized LDL immune complexes: role in phagocytosis and cytokine release
- PMID: 22236141
- PMCID: PMC3372755
- DOI: 10.1111/j.1365-2567.2012.03552.x
Differential regulation of acid sphingomyelinase in macrophages stimulated with oxidized low-density lipoprotein (LDL) and oxidized LDL immune complexes: role in phagocytosis and cytokine release
Abstract
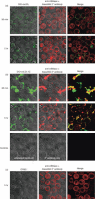
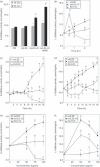
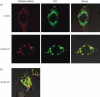
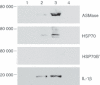
Oxidized low-density lipoprotein (oxLDL) and oxLDL-containing immune complexes (oxLDL-IC) contribute to the formation of lipid-laden macrophages (foam cells). Fcγ receptors mediate uptake of oxLDL-IC, whereas scavenger receptors internalize oxLDL. We have previously reported that oxLDL-IC, but not free oxLDL, activate macrophages and prolong their survival. Sphingomyelin is a major constituent of cell membranes and lipoprotein particles and acid sphingomyelinase (ASMase) hydrolyses sphingomyelin to generate the bioactive lipid ceramide. ASMase exists in two forms: lysosomal (L-ASMase) and secretory (S-ASMase). In this study we examined whether oxLDL and oxLDL-IC regulate ASMase differently, and whether ASMase mediates monocyte/macrophage activation and cytokine release. The oxLDL-IC, but not oxLDL, induced early and consistent release of catalytically active S-ASMase. The oxLDL-IC also consistently stimulated L-ASMase activity, whereas oxLDL induced a rapid transient increase in L-ASMase activity before it steadily declined below baseline. Prolonged exposure to oxLDL increased L-ASMase activity; however, activity remained significantly lower than that induced by oxLDL-IC. Further studies were aimed at defining the function of the activated ASMase. In response to oxLDL-IC, heat-shock protein 70B' (HSP70B') was up-regulated and localized with redistributed ASMase in the endosomal compartment outside the lysosome. Treatment with oxLDL-IC induced the formation and release of HSP70-containing and IL-1β-containing exosomes via an ASMase-dependent mechanism. Taken together, the results suggest that oxLDL and oxLDL-IC differentially regulate ASMase activity, and the pro-inflammatory responses to oxLDL-IC are mediated by prolonged activation of ASMase. These findings may contribute to increased understanding of mechanisms mediating macrophage involvement in atherosclerosis.
© 2012 The Authors. Immunology © 2012 Blackwell Publishing Ltd.
Figures



Similar articles
-
Differential trafficking of oxidized LDL and oxidized LDL immune complexes in macrophages: impact on oxidative stress.PLoS One. 2010 Sep 2;5(9):e12534. doi: 10.1371/journal.pone.0012534. PLoS One. 2010. PMID: 20824093 Free PMC article.
-
Heat shock protein 70B' (HSP70B') expression and release in response to human oxidized low density lipoprotein immune complexes in macrophages.J Biol Chem. 2010 May 21;285(21):15985-93. doi: 10.1074/jbc.M110.113605. Epub 2010 Mar 26. J Biol Chem. 2010. PMID: 20348092 Free PMC article.
-
Acid sphingomyelinase recruits palmitoylated CD36 to membrane rafts and enhances lipid uptake.J Biol Chem. 2025 Jun;301(6):110213. doi: 10.1016/j.jbc.2025.110213. Epub 2025 May 8. J Biol Chem. 2025. PMID: 40348192 Free PMC article.
-
Acid sphingomyelinase in macrophage biology.Cell Mol Life Sci. 2011 Oct;68(20):3293-305. doi: 10.1007/s00018-011-0686-6. Epub 2011 May 2. Cell Mol Life Sci. 2011. PMID: 21533981 Free PMC article. Review.
-
Oxidized LDL-induced endolysosomal phospholipidosis and enzymatically modified LDL-induced foam cell formation determine specific lipid species modulation in human macrophages.Chem Phys Lipids. 2011 Sep;164(6):479-87. doi: 10.1016/j.chemphyslip.2011.06.001. Epub 2011 Jun 12. Chem Phys Lipids. 2011. PMID: 21683693 Review.
Cited by
-
Tesaglitazar ameliorates non-alcoholic fatty liver disease and atherosclerosis development in diabetic low-density lipoprotein receptor-deficient mice.Exp Ther Med. 2012 Dec;4(6):987-992. doi: 10.3892/etm.2012.713. Epub 2012 Sep 17. Exp Ther Med. 2012. PMID: 23226761 Free PMC article.
-
The Multifunctionality of CD36 in Diabetes Mellitus and Its Complications-Update in Pathogenesis, Treatment and Monitoring.Cells. 2020 Aug 11;9(8):1877. doi: 10.3390/cells9081877. Cells. 2020. PMID: 32796572 Free PMC article. Review.
-
Medial calcification in the arterial wall of smooth muscle cell-specific Smpd1 transgenic mice: A ceramide-mediated vasculopathy.J Cell Mol Med. 2020 Jan;24(1):539-553. doi: 10.1111/jcmm.14761. Epub 2019 Nov 19. J Cell Mol Med. 2020. PMID: 31743567 Free PMC article.
-
Immune complex formation in human diabetic retina enhances toxicity of oxidized LDL towards retinal capillary pericytes.J Lipid Res. 2014 May;55(5):860-9. doi: 10.1194/jlr.M045401. Epub 2014 Mar 10. J Lipid Res. 2014. PMID: 24616481 Free PMC article.
-
Aging and serum exomiR content in women-effects of estrogenic hormone replacement therapy.Sci Rep. 2017 Feb 14;7:42702. doi: 10.1038/srep42702. Sci Rep. 2017. PMID: 28195143 Free PMC article.
References
-
- Krieger M, Herz J. Structures and functions of multiligand lipoprotein receptors: macrophage scavenger receptors and LDL receptor-related protein (LRP) Annu Rev Biochem. 1994;63:601–37. - PubMed
-
- Jerome WG, Cash C, Webber R, Horton R, Yancey PG. Lysosomal lipid accumulation from oxidized low density lipoprotein is correlated with hypertrophy of the Golgi apparatus and trans-Golgi network. J Lipid Res. 1998;39:1362–71. - PubMed
-
- Virella G, Koskinen S, Krings G, Onorato JM, Thorpe SR, Lopes-Virella M. Immunochemical characterization of purified human oxidized low-density lipoprotein antibodies. Clin Immunol. 2000;95:135–44. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

