Analysis of the interactions of viral and cellular factors with human cytomegalovirus lytic origin of replication, oriLyt
- PMID: 22236369
- PMCID: PMC3288578
- DOI: 10.1016/j.virol.2011.12.010
Analysis of the interactions of viral and cellular factors with human cytomegalovirus lytic origin of replication, oriLyt
Abstract
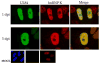
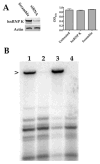
Human cytomegalovirus transient lytic DNA replication relies on the cis-acting element oriLyt, six viral-encoded core proteins, the proposed DNA replication initiator protein UL84, IE2, IRS1 and the gene products from the UL112/113 loci. In an effort to elucidate cellular and viral-encoded factors that may play a role in oriLyt-dependent replication we used DNA-affinity purification and mass spectrometry to isolate and identify several previously unknown cellular and viral factors that interact with HCMV oriLyt DNA. These proteins include the multifunctional hnRNP-K, BUB3, HMGB1, PTB-1, UL83, UL112/113, and IRS1. Chromatin immunoprecipitation (ChIP) assays confirmed an interaction of several of these factors with oriLyt. Co-immunoprecipitation experiments detected an interaction between UL84 and hnRNP-K in infected and transfected cells. Knockdown of hnRNP K expression by siRNA inhibited the amplification of oriLyt in the transient assay. Together, these data suggest a possible regulatory role in DNA replication for several previously unidentified viral and cellular factors.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures



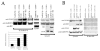
Similar articles
-
Evidence that the UL84 gene product of human cytomegalovirus is essential for promoting oriLyt-dependent DNA replication and formation of replication compartments in cotransfection assays.J Virol. 1996 Nov;70(11):7398-413. doi: 10.1128/JVI.70.11.7398-7413.1996. J Virol. 1996. PMID: 8892858 Free PMC article.
-
Nucleocytoplasmic shuttling of human cytomegalovirus UL84 is essential for virus growth.J Virol. 2010 Sep;84(17):8484-94. doi: 10.1128/JVI.00738-10. Epub 2010 Jun 23. J Virol. 2010. PMID: 20573826 Free PMC article.
-
Human cytomegalovirus UL84 oligomerization and heterodimerization domains act as transdominant inhibitors of oriLyt-dependent DNA replication: evidence that IE2-UL84 and UL84-UL84 interactions are required for lytic DNA replication.J Virol. 2004 Sep;78(17):9203-14. doi: 10.1128/JVI.78.17.9203-9214.2004. J Virol. 2004. PMID: 15308715 Free PMC article.
-
Nuts and bolts of human cytomegalovirus lytic DNA replication.Curr Top Microbiol Immunol. 2008;325:153-66. doi: 10.1007/978-3-540-77349-8_9. Curr Top Microbiol Immunol. 2008. PMID: 18637505 Review.
-
Human cytomegalovirus riding the cell cycle.Med Microbiol Immunol. 2015 Jun;204(3):409-19. doi: 10.1007/s00430-015-0396-z. Epub 2015 Mar 17. Med Microbiol Immunol. 2015. PMID: 25776080 Review.
Cited by
-
Inclusion of Antibodies to Cell Culture Media Preserves the Integrity of Genes Encoding RL13 and the Pentameric Complex Components During Fibroblast Passage of Human Cytomegalovirus.Viruses. 2019 Mar 5;11(3):221. doi: 10.3390/v11030221. Viruses. 2019. PMID: 30841507 Free PMC article.
-
Extracellular HMGB1 as Inflammatory Mediator in the Progression of Mycoplasma Gallisepticum Infection.Cells. 2022 Sep 9;11(18):2817. doi: 10.3390/cells11182817. Cells. 2022. PMID: 36139393 Free PMC article.
-
Transcription of true late (γ2) cytomegalovirus genes requires UL92 function that is conserved among beta- and gammaherpesviruses.J Virol. 2014 Jan;88(1):120-30. doi: 10.1128/JVI.02983-13. Epub 2013 Oct 16. J Virol. 2014. PMID: 24131715 Free PMC article.
-
Dynamic and nucleolin-dependent localization of human cytomegalovirus UL84 to the periphery of viral replication compartments and nucleoli.J Virol. 2014 Oct;88(20):11738-47. doi: 10.1128/JVI.01889-14. Epub 2014 Jul 30. J Virol. 2014. PMID: 25078694 Free PMC article.
-
β-catenin has potential effects on the expression, subcellular localization, and release of high mobility group box 1 during bovine herpesvirus 1 productive infection in MDBK cell culture.Virulence. 2021 Dec;12(1):1345-1361. doi: 10.1080/21505594.2021.1926409. Virulence. 2021. PMID: 34008469 Free PMC article.
References
-
- Bomsztyk K, Denisenko O, Ostrowski J. hnRNP K: one protein multiple processes. Bioessays. 2004;26(6):629–38. - PubMed
-
- Colletti KS, Xu Y, Cei SA, Tarrant M, Pari GS. Human cytomegalovirus UL84 oligomerization and heterodimerization domains act as transdominant inhibitors of oriLyt-dependent DNA replication: evidence that IE2-UL84 and UL84-UL84 interactions are required for lytic DNA replication. J Virol. 2004;78(17):9203–14. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

