Effects of glucagon-like peptide-1 receptor agonists on weight loss: systematic review and meta-analyses of randomised controlled trials
- PMID: 22236411
- PMCID: PMC3256253
- DOI: 10.1136/bmj.d7771
Effects of glucagon-like peptide-1 receptor agonists on weight loss: systematic review and meta-analyses of randomised controlled trials
Abstract
Objective: To determine whether treatment with agonists of glucagon-like peptide-1 receptor (GLP-1R) result in weight loss in overweight or obese patients with or without type 2 diabetes mellitus.
Design: Systematic review with meta-analyses.
Data sources: Electronic searches (Cochrane Library, Medline, Embase, and Web of Science) and manual searches (up to May 2011). Review methods Randomised controlled trials of adult participants with a body mass index of 25 or higher; with or without type 2 diabetes mellitus; and who received exenatide twice daily, exenatide once weekly, or liraglutide once daily at clinically relevant doses for at least 20 weeks. Control interventions assessed were placebo, oral antidiabetic drugs, or insulin.
Data extraction: Three authors independently extracted data. We used random effects models for the primary meta-analyses. We also did subgroup, sensitivity, regression, and sequential analyses to evaluate sources of intertrial heterogeneity, bias, and the robustness of results after adjusting for multiple testing and random errors.
Results: 25 trials were included in the analysis. GLP-1R agonist groups achieved a greater weight loss than control groups (weighted mean difference -2.9 kg, 95% confidence interval -3.6 to -2.2; 21 trials, 6411 participants). We found evidence of intertrial heterogeneity, but no evidence of bias or small study effects in regression analyses. The results were confirmed in sequential analyses. We recorded weight loss in the GLP-1R agonist groups for patients without diabetes (-3.2 kg, -4.3 to -2.1; three trials) as well as patients with diabetes (-2.8 kg, -3.4 to -2.3; 18 trials). In the overall analysis, GLP-1R agonists had beneficial effects on systolic and diastolic blood pressure, plasma concentrations of cholesterol, and glycaemic control, but did not have a significant effect on plasma concentrations of liver enzymes. GLP-1R agonists were associated with nausea, diarrhoea, and vomiting, but not with hypoglycaemia.
Conclusions: The present review provides evidence that treatment with GLP-1R agonists leads to weight loss in overweight or obese patients with or without type 2 diabetes mellitus.
Conflict of interest statement
Competing interests: All authors have completed the Unified Competing Interest form at
Figures


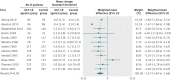
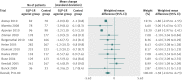
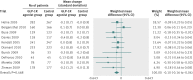


Comment in
-
Glucagon-like peptide-1 agonists.BMJ. 2012 Jan 10;344:d7282. doi: 10.1136/bmj.d7282. BMJ. 2012. PMID: 22236410 No abstract available.
-
Pharmacotherapy: GLP-1R agonists--the new weapon against obesity?Nat Rev Endocrinol. 2012 Jan 31;8(4):196. doi: 10.1038/nrendo.2012.13. Nat Rev Endocrinol. 2012. PMID: 22290361 No abstract available.
-
Utility of GLP-1R agonists in diabetes requires long term study.BMJ. 2012 Feb 28;344:e1451; author reply e1459. doi: 10.1136/bmj.e1451. BMJ. 2012. PMID: 22374904 No abstract available.
-
GLP-1R agonists are not a quick fix for weight loss.BMJ. 2012 Feb 28;344:e1455; author reply e1459. doi: 10.1136/bmj.e1455. BMJ. 2012. PMID: 22374905 No abstract available.
References
-
- Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9·1 million participants. Lancet 2011;377:557-67. - PMC - PubMed
-
- World Health Organization. Obesity and overweight. 2011. www.who.int/mediacentre/factsheets/fs311/en/.
-
- Franz MJ, VanWormer JJ, Crain AL, Boucher JL, Histon T, Caplan W, et al. Weight-loss outcomes: a systematic review and meta-analysis of weight-loss clinical trials with a minimum 1-year follow-up. J Am Diet Assoc 2007;107:1755-67. - PubMed
-
- Gourlan MJ, Trouilloud DO, Sarrazin PG. Interventions promoting physical activity among obese populations: a meta-analysis considering global effect, long-term maintenance, physical activity indicators and dose characteristics. Obes Rev 2011;12:e633-45. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
