Altered CXCR3 isoform expression regulates prostate cancer cell migration and invasion
- PMID: 22236567
- PMCID: PMC3320557
- DOI: 10.1186/1476-4598-11-3
Altered CXCR3 isoform expression regulates prostate cancer cell migration and invasion
Abstract
Background: Carcinoma cells must circumvent the normally suppressive signals to disseminate. While often considered 'stop' signals for adherent cells, CXCR3-binding chemokines have recently been correlated positively with cancer progression though the molecular basis remains unclear.
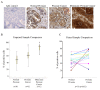
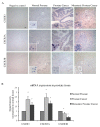
Results: Here, we examined the expression and function of two CXCR3 variants in human prostate cancer biopsies and cell lines. Globally, both CXCR3 mRNA and protein were elevated in localized and metastatic human cancer biopsies compared to normal. Additionally, CXCR3A mRNA level was upregulated while CXCR3B mRNA was downregulated in these prostate cancer specimens. In contrast to normal prostate epithelial cells (RWPE-1), CXCR3A was up to half the receptor in the invasive and metastatic DU-145 and PC-3 prostate cancer cells, but not in the localized LNCaP cells. Instead of inhibiting cell migration as in RWPE-1 cells, the CXCR3 ligands CXCL4/PF4 and CXCL10/IP10 promoted cell motility and invasiveness in both DU-145 and PC-3 cells via PLCβ3 and μ-calpain activation. CXCR3-mediated diminution of cell motility in RWPE-1 cells is likely a result of cAMP upregulation and m-calpain inhibition via CXCR3B signal transduction. Interestingly, overexpression of CXCR3B in DU-145 cells decreased cell movement and invasion.
Conclusion: These data suggest that the aberrant expression of CXCR3A and down-regulation of CXCR3B may switch a progression "stop" to a "go" signal to promote prostate tumor metastasis via stimulating cell migration and invasion.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

