Selective optogenetic stimulation of cholinergic axons in neocortex
- PMID: 22236708
- PMCID: PMC3331667
- DOI: 10.1152/jn.00870.2011
Selective optogenetic stimulation of cholinergic axons in neocortex
Abstract
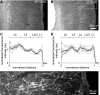
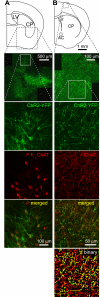
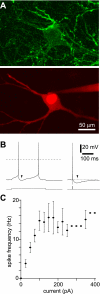
Acetylcholine profoundly affects neocortical function, being involved in arousal, attention, learning, memory, sensory and motor function, and plasticity. The majority of cholinergic afferents to neocortex are from neurons in nucleus basalis. Nucleus basalis also contains projecting neurons that release other transmitters, including GABA and possibly glutamate. Hence, electrical stimulation of nucleus basalis evokes the release of a mixture of neurotransmitters in neocortex, and this lack of selectivity has impeded research on cholinergic signaling in neocortex. We describe a method for the selective stimulation of cholinergic axons in neocortex. We used the Cre-lox system and a viral vector to express the light-activated protein channelrhodopsin-2 in cholinergic neurons in nucleus basalis and their axons in neocortex. Labeled neurons depolarized on illumination with blue light but were otherwise unchanged. In anesthetized mice, illumination of neocortex desynchronized the local field potential, indicating that light evoked release of ACh. This novel technique will enable many new studies of the cellular, network, and behavioral physiology of ACh in neocortex.
Figures






Similar articles
-
Modulation of Specific Sensory Cortical Areas by Segregated Basal Forebrain Cholinergic Neurons Demonstrated by Neuronal Tracing and Optogenetic Stimulation in Mice.Front Neural Circuits. 2016 Apr 20;10:28. doi: 10.3389/fncir.2016.00028. eCollection 2016. Front Neural Circuits. 2016. PMID: 27147975 Free PMC article.
-
Neostriatal GABAergic Interneurons Mediate Cholinergic Inhibition of Spiny Projection Neurons.J Neurosci. 2016 Sep 7;36(36):9505-11. doi: 10.1523/JNEUROSCI.0466-16.2016. J Neurosci. 2016. PMID: 27605623 Free PMC article.
-
Optogenetic analysis of neuromuscular transmission in the colon of ChAT-ChR2-YFP BAC transgenic mice.Am J Physiol Gastrointest Liver Physiol. 2019 Nov 1;317(5):G569-G579. doi: 10.1152/ajpgi.00089.2019. Epub 2019 Aug 14. Am J Physiol Gastrointest Liver Physiol. 2019. PMID: 31411893 Free PMC article.
-
Cholinergic circuitry of the human nucleus basalis and its fate in Alzheimer's disease.J Comp Neurol. 2013 Dec 15;521(18):4124-44. doi: 10.1002/cne.23415. J Comp Neurol. 2013. PMID: 23852922 Free PMC article. Review.
-
Organization of the basal forebrain cholinergic system in the human brain.Handb Clin Neurol. 2025;211:11-21. doi: 10.1016/B978-0-443-19088-9.00009-3. Handb Clin Neurol. 2025. PMID: 40340056 Review.
Cited by
-
Discharge and Role of GABA Pontomesencephalic Neurons in Cortical Activity and Sleep-Wake States Examined by Optogenetics and Juxtacellular Recordings in Mice.J Neurosci. 2020 Jul 29;40(31):5970-5989. doi: 10.1523/JNEUROSCI.2875-19.2020. Epub 2020 Jun 23. J Neurosci. 2020. PMID: 32576622 Free PMC article.
-
Central Cholinergic Neurons Are Rapidly Recruited by Reinforcement Feedback.Cell. 2015 Aug 27;162(5):1155-68. doi: 10.1016/j.cell.2015.07.057. Cell. 2015. PMID: 26317475 Free PMC article.
-
Principles of designing interpretable optogenetic behavior experiments.Learn Mem. 2015 Mar 18;22(4):232-8. doi: 10.1101/lm.038026.114. Print 2015 Apr. Learn Mem. 2015. PMID: 25787711 Free PMC article. Review.
-
Parkinson's disease dementia: a neural networks perspective.Brain. 2015 Jun;138(Pt 6):1454-76. doi: 10.1093/brain/awv104. Epub 2015 Apr 16. Brain. 2015. PMID: 25888551 Free PMC article. Review.
-
Deep Brain Stimulation of the Nucleus Basalis of Meynert for Parkinson's Disease Dementia: A 36 Months Follow Up Study.Mov Disord Clin Pract. 2022 Jul 27;9(6):765-774. doi: 10.1002/mdc3.13510. eCollection 2022 Aug. Mov Disord Clin Pract. 2022. PMID: 35937485 Free PMC article.
References
-
- Aravanis AM, Wang LP, Zhang F, Meltzer LA, Mogri MZ, Schneider MB, Deisseroth K. An optical neural interface: in vivo control of rodent motor cortex with integrated fiberoptic and optogenetic technology. J Neural Eng 4: S143–S156, 2007 - PubMed
-
- Avendanǒ C, Umbriaco D, Dykes RW, Descarries L. Acetylcholine innervation of sensory and motor neocortical areas in adult cat: a choline acetyltransferase immunohistochemical study. J Chem Neuroanat 11: 113–130, 1996 - PubMed
-
- Ayling OG, Harrison TC, Boyd JD, Goroshkov A, Murphy TH. Automated light-based mapping of motor cortex by photoactivation of channelrhodopsin-2 transgenic mice. Nat Methods 6: 219–224, 2009 - PubMed
-
- Berg RW, Friedman B, Schroeder LF, Kleinfeld D. Activation of nucleus basalis facilitates cortical control of a brain stem motor program. J Neurophysiol 94: 699–711, 2005 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

