Up-regulation of 12(S)-lipoxygenase induces a migratory phenotype in colorectal cancer cells
- PMID: 22237009
- PMCID: PMC3314953
- DOI: 10.1016/j.yexcr.2011.12.017
Up-regulation of 12(S)-lipoxygenase induces a migratory phenotype in colorectal cancer cells
Abstract
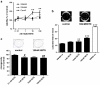
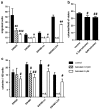
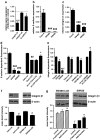
12(S)-Lipoxygenase (LOX) and its product 12(S)-hydroxyeicosatetraenic (HETE) acid have been implicated in angiogenesis and tumour invasion in several tumour types while their role in colorectal cancer progression has not yet been studied. We have analysed 12(S)-LOX expression in colorectal tumours and found gene expression up-regulated in colorectal cancer specimens for which the pathology report described involvement of inflammation. Using cell line models exposed to 12(S)-HETE or over-expressing 12(S)-LOX malignant cell growth as well as tumour cell migration was found to be stimulated. Specifically, Caco2 and SW480 cells over-expressing 12(S)-LOX formed fewer colonies from sparse cultures, but migrated better in filter-migration assays. SW480 LOX cells also had higher anchorage-independent growth capacity and a higher tendency to metastasise in vivo. Knock-down or inhibition of 12(S)-LOX inhibited cell migration and anchorage-independent growth in both 12(S)-LOX transfectants and SW620 cells that express high endogenous levels of 12(S)-LOX. On the cell surface E-cadherin and integrin-β1 expression were down-regulated in a 12(S)-LOX-dependent manner disturbing cell-cell interactions. The results demonstrate that 12(S)-LOX expression in inflammatory areas of colorectal tumours has the capacity to induce an invasive phenotype in colorectal cancer cells and could be targeted for therapy.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures



References
-
- Silverman E.S., Drazen J.M. The biology of 5-lipoxygenase: function, structure, and regulatory mechanisms. Proc. Assoc. Am. Physicians. 1999;111:525–536. - PubMed
-
- Funk C.D., Chen X.S., Johnson E.N., Zhao L. Lipoxygenase genes and their targeted disruption. Prostaglandins Other Lipid Mediat. 2002;68–69:303–312. - PubMed
-
- Kühn H., O'Donnell V.B. Inflammation and immune regulation by 12/15-lipoxygenases. Prog. Lipid Res. 2006;45:334–356. - PubMed
-
- Kuhn H. Biologic relevance of lipoxygenase isoforms in atherogenesis. Expert Rev. Cardiovasc. Ther. 2005;3:1099–1110. - PubMed
-
- Kuhn H., Walther M., Kuban R.J. Mammalian arachidonate 15-lipoxygenases structure, function, and biological implications. Prostaglandins Other Lipid Mediat. 2002;68–69:263–290. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

