Prevention of neonatal oxygen-induced brain damage by reduction of intrinsic apoptosis
- PMID: 22237207
- PMCID: PMC3270267
- DOI: 10.1038/cddis.2011.133
Prevention of neonatal oxygen-induced brain damage by reduction of intrinsic apoptosis
Abstract
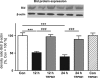
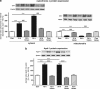
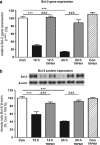
Within the last decade, it became clear that oxygen contributes to the pathogenesis of neonatal brain damage, leading to neurocognitive impairment of prematurely born infants in later life. Recently, we have identified a critical role for receptor-mediated neuronal apoptosis in the immature rodent brain. However, the contribution of the intrinsic apoptotic pathway accompanied by activation of caspase-2 under hyperoxic conditions in the neonatal brain still remains elusive. Inhibition of caspases appears a promising strategy for neuroprotection. In order to assess the influence of specific caspases on the developing brain, we applied a recently developed pentapeptide-based group II caspase inhibitor (5-(2,6-difluoro-phenoxy)-3(R,S)-(2(S)-(2(S)-(3-methoxycarbonyl-2(S)-(3-methyl-2(S)-((quinoline-2-carbonyl)-amino)-butyrylamino)propionylamino)3-methylbutyrylamino)propionylamino)-4-oxo-pentanoic acid methyl ester; TRP601). Here, we report that elevated oxygen (hyperoxia) triggers a marked increase in active caspase-2 expression, resulting in an initiation of the intrinsic apoptotic pathway with upregulation of key proteins, namely, cytochrome c, apoptosis protease-activating factor-1, and the caspase-independent protein apoptosis-inducing factor, whereas BH3-interacting domain death agonist and the anti-apoptotic protein B-cell lymphoma-2 are downregulated. These results coincide with an upregulation of caspase-3 activity and marked neurodegeneration. However, single treatment with TRP601 at the beginning of hyperoxia reversed the detrimental effects in this model. Hyperoxia-mediated neurodegeneration is supported by intrinsic apoptosis, suggesting that the development of highly selective caspase inhibitors will represent a potential useful therapeutic strategy in prematurely born infants.
Figures



References
-
- Johnson S, Wolke D, Hennessy E, Marlow N. Educational outcomes in extremely preterm children: neuropsychological correlates and predictors of attainment. Dev Neuropsychol. 2011;36:74–95. - PubMed
-
- Keller M, Felderhoff-Mueser U, Lagercrantz H, Dammann O, Marlow N, Hüppi P, et al. Policy benchmarking report on neonatal health and social policies in 13 European countries. Acta Paediatr. 2010;99:1624–1629. - PubMed
-
- Felderhoff-Mueser U, Sifringer M, Polley O, Dzietko M, Leineweber B, Mahler L, et al. Caspase-1-processed interleukins in hyperoxia-induced cell death in the developing brain. Ann Neurol. 2005;57:50–59. - PubMed
-
- Gerstner B, Sifringer M, Dzietko M, Schüller A, Lee J, Simons S, et al. Estradiol attenuates hyperoxia-induced cell death in the developing white matter. Ann Neurol. 2007;61:562–573. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

