Clostridium difficile toxins: mediators of inflammation
- PMID: 22237401
- PMCID: PMC3388264
- DOI: 10.1159/000332946
Clostridium difficile toxins: mediators of inflammation
Abstract
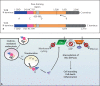
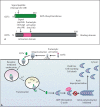
Clostridium difficile is a significant problem in hospital settings as the most common cause of nosocomial diarrhea worldwide. C. difficile infections (CDIs) are characterized by an acute intestinal inflammatory response with neutrophil infiltration. These symptoms are primarily caused by the glucosylating toxins, TcdA and TcdB. In the past decade, the frequency and severity of CDIs have increased markedly due to the emergence of so-called hypervirulent strains that overproduce cytotoxic glucosylating toxins relative to historical strains. In addition, these strains produce a third toxin, binary toxin or C. difficile transferase (CDT), that may contribute to hypervirulence. Both the glucosylating toxins and CDT covalently modify target cell proteins to cause disassembly of the actin cytoskeleton and induce severe inflammation. This review summarizes our current knowledge of the mechanisms by which glucosylating toxins and CDT disrupt target cell function, alter host physiology and stimulate immune responses.
Copyright © 2012 S. Karger AG, Basel.
Figures

References
-
- Carroll KC, Bartlett JG. Biology of Clostridium difficile: implications for epidemiology and diagnosis. Annu Rev Microbiol. 2011;65:501–521. - PubMed
-
- Rupnik M, Wilcox MH, Gerding DN. Clostridium difficile infection: new developments in epidemiology and pathogenesis. Nature Rev. 2009;7:526–536. - PubMed
-
- Gerding DN, Johnson S. Management of Clostridium difficile infection: thinking inside and outside the box. Clin Infect Dis. 2010;51:1306–1313. - PubMed
-
- Bartlett JG. Clinical practice: antibiotic-associated diarrhea. N Engl J Med. 2002;346:334–339. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

