Lactic acid restores skeletal muscle force in an in vitro fatigue model: are voltage-gated chloride channels involved?
- PMID: 22237405
- PMCID: PMC3330741
- DOI: 10.1152/ajpcell.00279.2011
Lactic acid restores skeletal muscle force in an in vitro fatigue model: are voltage-gated chloride channels involved?
Abstract
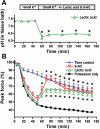
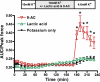
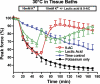
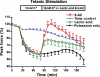
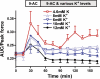
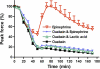
High interstitial K(+) concentration ([K(+)]) has been reported to impede normal propagation of electrical impulses along the muscle cell membrane (sarcolemma) and then also into the transverse tubule system; this is one considered underlying mechanism associated with the development of muscle fatigue. Interestingly, the extracellular buildup of lactic acid, once considered an additional cause for muscle fatigue, was recently shown to have force-restoring effects in such conditions. Specifically, it was proposed that elevated lactic acid (and intracellular acidosis) may lead to inhibition of voltage-gated chloride channels, thereby reestablishing better excitability of the muscle cell sarcolemma. In the present study, using an in vitro muscle contractile experimental setup to study functionally viable rectus abdominis muscle preparations obtained from normal swine, we examined the effects of 20 mM lactic acid and 512 μM 9-anthracenecarboxylic acid (9-AC; a voltage-gated chloride channel blocker) on the force recovery of K(+)-depressed (10 mM K(+)) twitch forces. We observed a similar muscle contractile restoration after both treatments. Interestingly, at elevated [K(+)], myotonia (i.e., hyperexcitability or afterdepolarizations), usually present in skeletal muscle with inherent or induced chloride channel dysfunctions, was not observed in the presence of either lactic acid or 9-AC. In part, these data confirm previous studies showing a force-restoring effect of lactic acid in high-[K(+)] conditions. In addition, we observed similar restorative effects of lactic acid and 9-AC, implicating a beneficial mechanism via voltage-gated chloride channel modulation.
Figures

References
-
- Allen DG, Lamb GD, Westerblad H. Skeletal muscle fatigue: cellular mechanisms. Physiol Rev 88: 287–332, 2008 - PubMed
-
- Allen DG, Lannergren J, Westerblad H. Muscle cell function during prolonged activity: cellular mechanisms of fatigue. Exp Physiol 80: 497–527, 1995 - PubMed
-
- Bandschapp O, Ginz HF, Soule CL, Girard T, Urwyler A, Iaizzo PA. In vitro effects of propofol and volatile agents on pharmacologically induced chloride channel myotonia. Anesthesiology 111: 584–590, 2009 - PubMed
-
- Bruton JD, Lannergren J, Westerblad H. Effects of CO2-induced acidification on the fatigue resistance of single mouse muscle fibers at 28°C. J Appl Physiol 85: 478–483, 1998 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

