Resolvin D1 prevents TNF-α-mediated disruption of salivary epithelial formation
- PMID: 22237406
- PMCID: PMC3361948
- DOI: 10.1152/ajpcell.00207.2011
Resolvin D1 prevents TNF-α-mediated disruption of salivary epithelial formation
Abstract
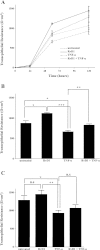
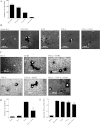
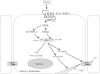
Sjögren's syndrome is a chronic autoimmune disorder characterized by inflammation of salivary glands resulting in impaired secretory function. Our present studies indicate that chronic exposure of salivary epithelium to TNF-α and/or IFN-γ alters tight junction integrity, leading to secretory dysfunction. Resolvins of the D-series (RvDs) are endogenous lipid mediators derived from DHA that regulate excessive inflammatory responses leading to resolution and tissue homeostasis. In this study, we addressed the hypothesis that activation of the RvD1 receptor ALX/FPR2 in salivary epithelium prevents and/or resolves the TNF-α-mediated disruption of acinar organization and enhances monolayer formation. Our results indicate that 1) the RvD1 receptor ALX/FPR2 is present in fresh, isolated cells from mouse salivary glands and in cell lines of salivary origin; and 2) the agonist RvD1 (100 ng/ml) abolished tight junction and cytoskeletal disruption caused by TNF-α and enhanced cell migration and polarity in salivary epithelium. These effects were blocked by the ALX/FPR2 antagonist butyloxycarbonyl-Phe-Leu-Phe-Leu-Phe. The ALX/FPR2 receptor signals via modulation of the phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathways since, in our study, blocking PI3K activation with LY294002, a potent and selective PI3K inhibitor, prevented RvD1-induced cell migration. Furthermore, Akt gene silencing with the corresponding siRNA almost completely blocked the ability of Par-C10 cells to migrate. Our findings suggest that RvD1 receptor activation promotes resolution of inflammation and tissue repair in salivary epithelium, which may have relevance in the restoration of salivary gland dysfunction associated with Sjögren's syndrome.
Figures





References
-
- Afonso PV, Ozden S, Prevost MC, Schmitt C, Seilhean D, Weksler B, Couraud PO, Gessain A, Romero IA, Ceccaldi PE. Human blood-brain barrier disruption by retroviral-infected lymphocytes: role of myosin light chain kinase in endothelial tight-junction disorganization. J Immunol 179: 2576–2583, 2007 - PubMed
-
- Alessi DR, Pearce LR, Garcia-Martinez JM. New insights into mTOR signaling: mTORC2 and beyond. Sci Signal 2: pe27, 2009 - PubMed
-
- Ariel A, Serhan CN. Resolvins and protectins in the termination program of acute inflammation. Trends Immunol 28: 176–183, 2007 - PubMed
-
- Arita M, Yoshida M, Hong S, Tjonahen E, Glickman JN, Petasis NA, Blumberg RS, Serhan CN. Resolvin E1, an endogenous lipid mediator derived from omega-3 eicosapentaenoic acid, protects against 2,4,6-trinitrobenzene sulfonic acid-induced colitis. Proc Natl Acad Sci USA 102: 7671–7676, 2005 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

