Successful immune tolerance induction to enzyme replacement therapy in CRIM-negative infantile Pompe disease
- PMID: 22237443
- PMCID: PMC3711224
- DOI: 10.1038/gim.2011.4
Successful immune tolerance induction to enzyme replacement therapy in CRIM-negative infantile Pompe disease
Abstract
Purpose: Infantile Pompe disease resulting from a deficiency of lysosomal acid α-glucosidase (GAA) requires enzyme replacement therapy (ERT) with recombinant human GAA (rhGAA). Cross-reactive immunologic material negative (CRIM-negative) Pompe patients develop high-titer antibody to the rhGAA and do poorly. We describe successful tolerance induction in CRIM-negative patients.
Methods: Two CRIM-negative patients with preexisting anti-GAA antibodies were treated therapeutically with rituximab, methotrexate, and gammaglobulins. Two additional CRIM-negative patients were treated prophylactically with a short course of rituximab and methotrexate, in parallel with initiating rhGAA.
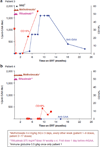
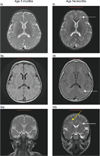
Results: In both patients treated therapeutically, anti-rhGAA was eliminated after 3 and 19 months. All four patients are immune tolerant to rhGAA, off immune therapy, showing B-cell recovery while continuing to receive ERT at ages 36 and 56 months (therapeutic) and 18 and 35 months (prophylactic). All patients show clinical response to ERT, in stark contrast to the rapid deterioration of their nontolerized CRIM-negative counterparts.
Conclusion: The combination of rituximab with methotrexate ± intravenous gammaglobulins (IVIG) is an option for tolerance induction of CRIM-negative Pompe to ERT when instituted in the naïve setting or following antibody development. It should be considered in other conditions in which antibody response to the therapeutic protein elicits robust antibody response that interferes with product efficacy.
Figures


References
-
- Kishnani PS, Howell RR. Pompe disease in infants and children. J Pediatr. 2004;144(suppl 5):S35–S43. - PubMed
-
- Amalfitano A, Bengur AR, Morse RP, et al. Recombinant human acid alpha-glucosidase enzyme therapy for infantile glycogen storage disease type II: results of a phase I/II clinical trial. Genet Med. 2001;3:132–138. - PubMed
-
- Kishnani PS, Corzo D, Nicolino M, et al. Recombinant human acid [alpha]-glucosidase: major clinical benefits in infantile-onset Pompe disease. Neurology. 2007;68:99–109. - PubMed
-
- Mendelsohn NJ, Messinger YH, Rosenberg AS, Kishnani PS. Elimination of antibodies to recombinant enzyme in Pompe’s disease. N Engl J Med. 2009;360:194–195. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

