Implementing screening for Lynch syndrome among patients with newly diagnosed colorectal cancer: summary of a public health/clinical collaborative meeting
- PMID: 22237445
- PMCID: PMC3762677
- DOI: 10.1038/gim.0b013e31823375ea
Implementing screening for Lynch syndrome among patients with newly diagnosed colorectal cancer: summary of a public health/clinical collaborative meeting
Abstract
Lynch syndrome is the most common cause of inherited colorectal cancer, accounting for approximately 3% of all colorectal cancer cases in the United States. In 2009, an evidence-based review process conducted by the independent Evaluation of Genomic Applications in Practice and Prevention Working Group resulted in a recommendation to offer genetic testing for Lynch syndrome to all individuals with newly diagnosed colorectal cancer, with the intent of reducing morbidity and mortality in family members. To explore issues surrounding implementation of this recommendation, the Centers for Disease Control and Prevention convened a multidisciplinary working group meeting in September 2010. This article reviews background information regarding screening for Lynch syndrome and summarizes existing clinical paradigms, potential implementation strategies, and conclusions which emerged from the meeting. It was recognized that widespread implementation will present substantial challenges, and additional data from pilot studies will be needed. However, evidence of feasibility and population health benefits and the advantages of considering a public health approach were acknowledged. Lynch syndrome can potentially serve as a model to facilitate the development and implementation of population-level programs for evidence-based genomic medicine applications involving follow-up testing of at-risk relatives. Such endeavors will require multilevel and multidisciplinary approaches building on collaborative public health and clinical partnerships.
Conflict of interest statement
DISCLOSURE
Heather Hampel, MS, has received an honorarium from Myriad Genetic Laboratories, Inc. to serve on a Lynch syndrome Advisory Panel. The other authors declare no conflict of interest.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or the National Cancer Institute.
Figures
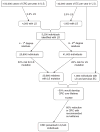
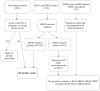

References
-
- U.S. Cancer Statistics Working Group. Department of Health and Human Services, Centers for Disease Control and Prevention, and National Cancer Institute; Atlanta, GA: 2010. [15 January 2011]. United States cancer statistics: 1999–2007 incidence and mortality web-based report. http://www.cdc.gov/uscs.
-
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. - PubMed
-
- Winawer S, Fletcher R, Rex D, et al. Colorectal cancer screening and surveillance: clinical guidelines and rationale-Update based on new evidence. Gastroenterology. 2003;124:544–560. - PubMed
-
- Lynch HT, de la Chapelle A. Hereditary colorectal cancer. N Engl J Med. 2003;348:919–932. - PubMed
-
- Hampel H, Frankel WL, Martin E, et al. Screening for the Lynch syndrome (hereditary nonpolyposis colorectal cancer) N Engl J Med. 2005;352:1851–1860. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

