Architecture of kangaroo rat inner medulla: segmentation of descending thin limb of Henle's loop
- PMID: 22237592
- PMCID: PMC3774486
- DOI: 10.1152/ajpregu.00549.2011
Architecture of kangaroo rat inner medulla: segmentation of descending thin limb of Henle's loop
Abstract
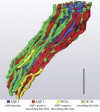
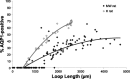
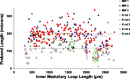
We hypothesize that the inner medulla of the kangaroo rat Dipodomys merriami, a desert rodent that concentrates its urine to more than 6,000 mosmol/kgH(2)O water, provides unique examples of architectural features necessary for production of highly concentrated urine. To investigate this architecture, inner medullary nephron segments in the initial 3,000 μm below the outer medulla were assessed with digital reconstructions from physical tissue sections. Descending thin limbs of Henle (DTLs), ascending thin limbs of Henle (ATLs), and collecting ducts (CDs) were identified by immunofluorescence using antibodies that label segment-specific proteins associated with transepithelial water flux (aquaporin 1 and 2, AQP1 and AQP2) and chloride flux (the chloride channel ClC-K1); all tubules and vessels were labeled with wheat germ agglutinin. In the outer 3,000 μm of the inner medulla, AQP1-positive DTLs lie at the periphery of groups of CDs. ATLs lie inside and outside the groups of CDs. Immunohistochemistry and reconstructions of loops that form their bends in the outer 3,000 μm of the inner medulla show that, relative to loop length, the AQP1-positive segment of the kangaroo rat is significantly longer than that of the Munich-Wistar rat. The length of ClC-K1 expression in the prebend region at the terminal end of the descending side of the loop in kangaroo rat is about 50% shorter than that of the Munich-Wistar rat. Tubular fluid of the kangaroo rat DTL may approach osmotic equilibrium with interstitial fluid by water reabsorption along a relatively longer tubule length, compared with Munich-Wistar rat. A relatively shorter-length prebend segment may promote a steeper reabsorptive driving force at the loop bend. These structural features predict functionality that is potentially significant in the production of a high urine osmolality in the kangaroo rat.
Figures




Similar articles
-
Architecture of vasa recta in the renal inner medulla of the desert rodent Dipodomys merriami: potential impact on the urine concentrating mechanism.Am J Physiol Regul Integr Comp Physiol. 2012 Oct 1;303(7):R748-56. doi: 10.1152/ajpregu.00300.2012. Epub 2012 Aug 22. Am J Physiol Regul Integr Comp Physiol. 2012. PMID: 22914749 Free PMC article.
-
Three-dimensional functional reconstruction of inner medullary thin limbs of Henle's loop.Am J Physiol Renal Physiol. 2004 Jan;286(1):F38-45. doi: 10.1152/ajprenal.00285.2003. Epub 2003 Sep 30. Am J Physiol Renal Physiol. 2004. PMID: 14519595
-
Three-dimensional lateral and vertical relationships of inner medullary loops of Henle and collecting ducts.Am J Physiol Renal Physiol. 2004 Oct;287(4):F767-74. doi: 10.1152/ajprenal.00122.2004. Epub 2004 Jun 8. Am J Physiol Renal Physiol. 2004. PMID: 15187004
-
Role of three-dimensional architecture in the urine concentrating mechanism of the rat renal inner medulla.Am J Physiol Renal Physiol. 2008 Nov;295(5):F1271-85. doi: 10.1152/ajprenal.90252.2008. Epub 2008 May 21. Am J Physiol Renal Physiol. 2008. PMID: 18495796 Free PMC article. Review.
-
Mechanisms to concentrate the urine: an opinion.Curr Opin Nephrol Hypertens. 2008 Jul;17(4):416-22. doi: 10.1097/MNH.0b013e328304b3f5. Curr Opin Nephrol Hypertens. 2008. PMID: 18660679 Review.
Cited by
-
A rather dry subject; investigating the study of arid-associated microbial communities.Environ Microbiome. 2020 Dec 1;15(1):20. doi: 10.1186/s40793-020-00367-6. Environ Microbiome. 2020. PMID: 33902728 Free PMC article. Review.
-
Characterization of a male reproductive transcriptome for Peromyscus eremicus (Cactus mouse).PeerJ. 2016 Oct 27;4:e2617. doi: 10.7717/peerj.2617. eCollection 2016. PeerJ. 2016. PMID: 27812417 Free PMC article.
-
Transepithelial water and urea permeabilities of isolated perfused Munich-Wistar rat inner medullary thin limbs of Henle's loop.Am J Physiol Renal Physiol. 2014 Jan 1;306(1):F123-9. doi: 10.1152/ajprenal.00491.2013. Epub 2013 Nov 6. Am J Physiol Renal Physiol. 2014. PMID: 24197065 Free PMC article.
-
Architecture of the human renal inner medulla and functional implications.Am J Physiol Renal Physiol. 2015 Oct 1;309(7):F627-37. doi: 10.1152/ajprenal.00236.2015. Epub 2015 Aug 19. Am J Physiol Renal Physiol. 2015. PMID: 26290371 Free PMC article.
-
Multiomic analysis of the Arabian camel (Camelus dromedarius) kidney reveals a role for cholesterol in water conservation.Commun Biol. 2021 Jun 23;4(1):779. doi: 10.1038/s42003-021-02327-3. Commun Biol. 2021. PMID: 34163009 Free PMC article.
References
-
- Akizuki N, Uchida S, Sasaki S, Marumo F. Impaired solute accumulation in inner medulla of Clcnk1−/− mice kidney. Am J Physiol Renal Physiol 280: F79–F87, 2001 - PubMed
-
- Bankir L, De Rouffignac C. Urinary concentrating ability: insights from comparative anatomy. Am J Physiol Regul Integr Comp Physiol 249: R643–R666, 1985 - PubMed
-
- Bankir L, Kriz W. Adaptation of the kidney to protein intake and to urine concentrating activity: similar consequences in health and CRF. Kidney Int 47: 7–24, 1995 - PubMed
-
- Barrett JM, Kriz W, Kaissling B, De Rouffignac C. The ultrastructure of the nephrons of the desert rodent (Psammonys obesus) kidney. II. Thin limbs of Henle of long-looped nephrons. Am J Anat 151: 499–514, 1978 - PubMed
-
- Beuchat CA. Body size, medullary thickness, and urine concentrating ability in mammals. Am J Physiol Regul Integr Comp Physiol 258: R298–R308, 1990 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

